Apply to immediate openings at Sanolet Lifecare, Lupin, RPG Life Sciences, Theon Pharmaceuticals & Swiss Pharma — walk-ins and online applications for Production, QC (Chemical & Micro), Stores, Marketing (B.Sc/B.Pharm), Strategy (MBA), Maintenance, Formulation & ITI roles. Interview dates Nov 16–21, 2025 across Ahmedabad, Bangalore, Hyderabad, Delhi, Chennai, Mumbai, Nalagarh, Thane & Vatva GIDC.
Sanolet Lifecare, Lupin, RPG Life Sciences, Theon & Swiss Pharma
This consolidated hiring bulletin highlights active recruitment drives and walk-ins across multiple Indian pharmaceutical companies in November 2025. If you are a fresher or experienced professional in Production, Quality Control (Chemical & Microbiology), Stores & Inventory, Marketing (B.Sc/B.Pharm), Strategy & Business Development (MBA), Maintenance, Formulation & ITI roles, this guide provides exact job profiles, responsibilities, skills required, benefits, venue and application instructions so you can prepare and apply immediately.
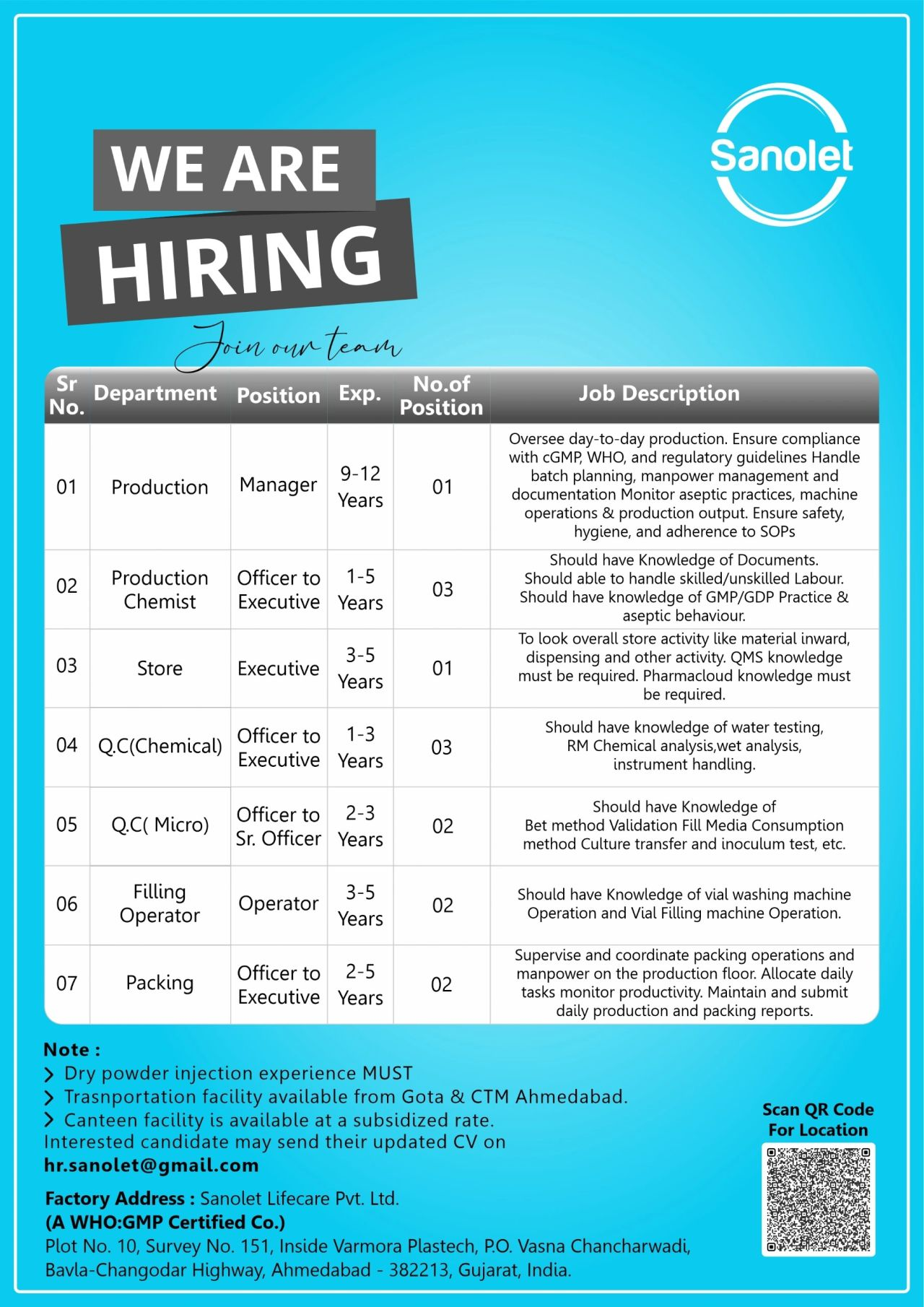
1. Sanolet Lifecare — Immediate Openings (Dry Powder Injections, Aseptic Production)
Company: Sanolet Lifecare Pvt. Ltd.
Location: Plot No.10, Survey No.151, Inside Varmora Plastech, P.O. Vasna Chancharwadi, Bavla-Changodar Highway, Ahmedabad – 382213, Gujarat, India
Work Type: On-site (Sterile/Dry Powder Injections)
Experience Range: 1–12 years (varies by role)
Sanolet Lifecare is expanding its Ahmedabad sterile injectables facility and recruiting across production, QC (chemical & micro), stores and packing. The focus is on candidates experienced in dry powder injection manufacturing, aseptic techniques, vial washing & filling, and strict cGMP/WHO-GMP compliance. These roles suit both professionals who want to deepen technical skills in sterile injectable processes and freshers with targeted internships or training.
Open Positions & Experience
- Production Manager — 9–12 years (oversee dry powder injection operations, batch release, compliance)
- Production Chemist (Officer/Executive) — 1–5 years (process handling, batch records, document control)
- Store Executive — 3–5 years (material inward, dispensing, inventory, ERP familiarity)
- QC (Chemical) — Officer/Executive — 1–3 years (HPLC, wet chemistry, RM analysis)
- QC (Micro) — Officer/Sr. Officer — 2–3 years (BET, sterility, media fill, environmental monitoring)
- Filling Operator — 3–5 years (vial washing/filling machine operation, aseptic behavior)
- Packing Officer/Executive — 2–5 years (secondary packaging supervision, reporting)
Key Skills & Certifications
- Hands-on experience with dry powder injection processes and sterile filling.
- Deep understanding of cGMP, WHO guidelines, GMP/GDP and QMS.
- Experience with HPLC, wet chemical analysis, BET validation, media fill and microbial validations.
- Practical knowledge of vial washing, filling and packing machines; aseptic techniques and gowning procedures.
- Good documentation habits: batch records, deviation management, CAPA and change control.
- Experience with Pharma ERP/PharmaCloud or material management systems is preferred.
Core Responsibilities
- Plan, execute and monitor production batches ensuring regulatory compliance.
- Supervise manpower, batch allocation and timely completion of production cycles.
- Coordinate inbound materials, dispensing and maintain accurate inventory records.
- Perform routine and stability water/chemical testing; report and escalate findings.
- Conduct and document microbial validations, media fills and environmental monitoring.
- Operate and troubleshoot filling lines and ensure aseptic behaviors on the floor.
- Supervise packing lines and produce daily shift reports for management.
Benefits & Employee Perks
- Company transport from Gota & CTM, Ahmedabad.
- Subsidized canteen enabling affordable meals on-site.
- WHO-GMP certified operations with scope for career growth.
- Training programs on advanced aseptic operations and QMS best practices.
- Supportive work culture prioritizing safety, upskilling and innovation.
How to Apply
Email your CV highlighting dry powder injection or injectable experience to: hr.sanolet@gmail.com
Use subject line: “Position – [Your Name]”. Include contact number, current CTC (if applicable), notice period and availability for immediate joining.
2. Lupin Limited — Walk in Interviews for Marketing Executives & Trainees (Women Candidates Encouraged)
Company: Lupin Limited
Positions: Marketing Executives & Marketing Trainees (Field Sales)
Eligibility: B.Sc. or B.Pharm (Freshers & Experienced) — women candidates encouraged
Cities & Dates:
- Bangalore: 19 Nov 2025 — Lupin Ltd, Petrotech House, Mysore Road
- Hyderabad: 20 Nov 2025 — Sri Business Center, Banjara Hills
- Delhi: 20 Nov 2025 — Agarwal Plaza, Rohini
- Chennai: 20 Nov 2025 — Cenotaph Road, Alwarpet
- Mumbai: 21 Nov 2025 — Rupa Sapphire, Sanpada, Navi Mumbai
Time: 09:30 AM – 05:00 PM (standard walk-in timing)
Contact Email: saleshr@lupin.com
Overview
Lupin is running multi-city walk-in interviews to strengthen its sales force for branded formulation promotion. The company seeks enthusiastic field sales professionals and trainees with strong communication skills, territory ownership and the ability to build relationships with healthcare providers.
Roles & Responsibilities
- Marketing Executive: Territory promotion of Lupin branded formulations, achieve monthly sales targets, doctor and pharmacy engagement, and reporting market feedback.
- Marketing Trainee: On-the-job training in pharma sales, product detailing, territory mapping and learning regulatory-compliant promotion.
Requirements & Ideal Candidate Profile
- Qualification: B.Sc. or B.Pharm.
- Language: Fluency in English + local language of posting city.
- Age Preference: Below 28 years for trainee roles (women candidates preferred for certain drives).
- Good communication, interpersonal and territory management skills.
- Willingness to travel and work field hours.
Benefits
- Structured training and mentorship accelerating sales career growth.
- Competitive salary with attractive incentives and performance-based bonuses.
- Exposure to national level pharma markets and potential international opportunities as career progresses.
How to Apply
- Walk-in: Carry updated resume, educational certificates, ID proof and any reference letters.
- Email: Send resume to saleshr@lupin.com with subject “Marketing Executive/Trainee – [City] Application”.
3. RPG Life Sciences — Strategy & Business Development Project Associate (API Division)
Company: RPG Life Sciences Ltd.
Location: RPGLS Thane API Factory (Turbe), Thane, Maharashtra
Role: Strategy & Business Development — Project Associate (API Business)
Eligibility: MBA (pursuing or passed out) with 6–12 months of relevant work experience
Apply By: 18 Nov 2025
Email: recruitments@rpgis.com
Role Summary
RPG Life Sciences seeks a Strategy & Business Development Project Associate to support the API (Active Pharmaceutical Ingredients) division. This is a high-learning role suited to early-career MBAs who want exposure to market analysis, deal-sourcing, valuation, GTM planning and cross-functional execution in the API export and domestic markets.
Key Responsibilities
- Conduct market research and competitive analysis in API segments.
- Identify potential molecules and markets for expansion; prepare business cases with ROI analysis.
- Support due diligence, target profiling and SWOT/financial analysis.
- Prepare strategic decks and reports for executive leadership.
- Coordinate with regulatory, technical and commercial teams to align GTM strategies.
- Monitor global demand-supply dynamics and pricing trends to inform strategy.
Skills Required
- Strong analytical and financial modeling skills.
- Proficiency with data tools, Excel, and presentation software.
- Good communication and stakeholder management capabilities.
- Awareness of regulatory frameworks governing APIs and exports.
- Ability to build structured business cases and measurable KPIs.
Benefits
- Exposure to API strategy and global trade in a ₹3,500+ crore pharma company.
- Fast learning curve, mentorship from senior leadership and high visibility projects.
- Competitive compensation and performance incentives.
- Opportunity to work on cross-border initiatives and global market expansion.
How to Apply
Email recruitments@rpgis.com with your resume and a brief cover note highlighting MBA projects or work that demonstrates analytical capability, strategic thinking, and availability for immediate joining.
4. Theon Pharmaceuticals — QC, Production, Maintenance, Microbiology & Warehouse
Company: Theon Pharmaceuticals Ltd.
Location: Nalagarh, Solan, Himachal Pradesh
Experience Range: 1–10 years (varies by role)
Compensation: Competitive; selected roles advertised with salaries up to INR 35,000
Open Roles
- Quality Control Officer / Sr. Officer: HPLC & GLP testing (1–3 years) — B.Sc / M.Sc (Chemistry)
- Production Officer / Compression & Coating Operators: 2–5 years — B.Pharm / Diploma
- Maintenance (Mechanical/Electrical/HVAC): 2–10 years — B.Tech/Diploma
- Microbiology / QA Officer: 3–4 years — M.Sc Microbiology / B.Pharm
- Warehouse Officer: 2–5 years — Diploma in Supply Chain or equivalent
Skills & Responsibilities
- Conduct analytical HPLC and GLP-compliant testing; maintain accurate QC documentation.
- Manage tablet and capsule compression, coating and packing operations with production KPIs.
- Execute validation, IPQA, AQA activities and ensure compliance with QMS.
- Maintain HVAC and utility systems to support sterile and non-sterile operations.
- Perform microbiological testing and ensure sterility/limit testing for DPI and injectables.
- Manage RM/PM inventory, issue/receipt and documentation in warehouse operations.
Benefits
- Competitive salary packages (indicative 18k–35k depending on role & experience).
- Career progression in a WHO-GMP certified manufacturing facility with export exposure.
- Training and skill development opportunities.
- Contact for queries: +91 77173 04694 or +91 78329 22841
- Apply via email: hr2@theonpharma.com or hr4@theonpharma.com

5. Swiss Pharma Pvt. Ltd. — Freshers & Experienced Hiring (OSD & Injectables)
Company: Swiss Pharma Pvt. Ltd.
Location: 3709 GIDC, Phase IV, Vatva GIDC, Ahmedabad – 382445, Gujarat, India
Experience: Fresher to 5 years (varies)
Roles: Production Chemist, Formulation & Development Officer, QC (Micro), QC, ITI Fitter
Role Breakdown
- Production Chemist (Fresher) — B.Pharm/M.Pharm/M.Sc; assist in formulation and production activities.
- Formulation & Development Officer — 1–2 years; optimize oral solid dosage (OSD) formulations.
- QC (Micro) & QC Analyst — 2–5 years; microbiological testing and analytical QA.
- ITI Fitter — 2–3 years; operate autoclave, vial washing and mechanical maintenance.
Skills & Responsibilities
- Support R&D pilots and scale-up of oral solid dose and injectable formulations.
- Execute microbial limit tests, sterility assays, and analytical testing per SOPs.
- Operate and maintain autoclaves, vial washers and related equipment.
- Maintain accurate batch documentation and adhere to GMP norms.
- Participate in validation activities and improvement initiatives.
Benefits & Apply
- Clear career progression, on-the-job training and competitive pay.
- For applications: Email info@swisspharma.in or maulik.t@swisspharma.in. Call 079 4008 3418 for queries. Include role in subject line and specify experience and relevant instruments handled.
Preparing for Walk ins & Onsite Interviews
- Documents to Carry
- Updated resume (one or two copies)
- Photo ID (Aadhar/Passport/Driving License)
- Educational Certificates (original + photocopies)
- Experience Letters and Last 3 Months’ Payslips (if experienced)
- Passport-sized photographs
- Bank details or a recent bank statement (if asked for verification)
- Interview Readiness
- Brush up on GMP, QMS, and role-specific SOPs.
- Be ready to discuss batch records, deviation handling, CAPA and validation experiences.
- For sales roles, prepare a short territory pitch and examples of how you met targets or increased prescriptions.
- Technical Prep
- QC Candidates: Review HPLC troubleshooting, analytical calculations, and GLP practices.
- Microbiology: Demonstrate knowledge of BET, sterility testing, media preparation, and environmental monitoring.
- Production/Maintenance: Explain hands-on experience with machines (compression, coating, filling, vial washing) and basic mechanical/electrical troubleshooting.
- Soft Skills
- Effective communication, time management, teamwork, and adherence to safety protocols are mandatory.
- For field sales roles, highlight local language fluency and stakeholder engagement skills.