💉 Pharma Walk-In Drives: USV, Anbuva, Axa, BioMatrix Hiring QC, Production, Regulatory Affairs
The pharmaceutical industry, a cornerstone of global healthcare, continues its dynamic expansion in India, creating abundant career pathways for skilled professionals. This comprehensive guide details the major walk-in interview drives scheduled for mid-December 2025 by leading pharmaceutical companies, including USV Private Limited, Anbuva Pharma, Axa Parenterals, BioMatrix Healthcare, and Parexel. These opportunities span critical functional areas such as Quality Control (QC), Quality Assurance (QA), Production, Regulatory Affairs, Engineering, and support services, catering to candidates with expertise in Oral Solid Dosages (OSD), Active Pharmaceutical Ingredients (API), and Parenteral/Injectable formulations. Prospective candidates seeking roles in highly regulated environments (USFDA, MHRA, EU-approved facilities) across locations like Daman, Mehsana, Ahmedabad, Roorkee, and Bengaluru are urged to review the specific requirements and dates outlined below.
1. USV Pvt. Ltd – Mega Walk-In Drive
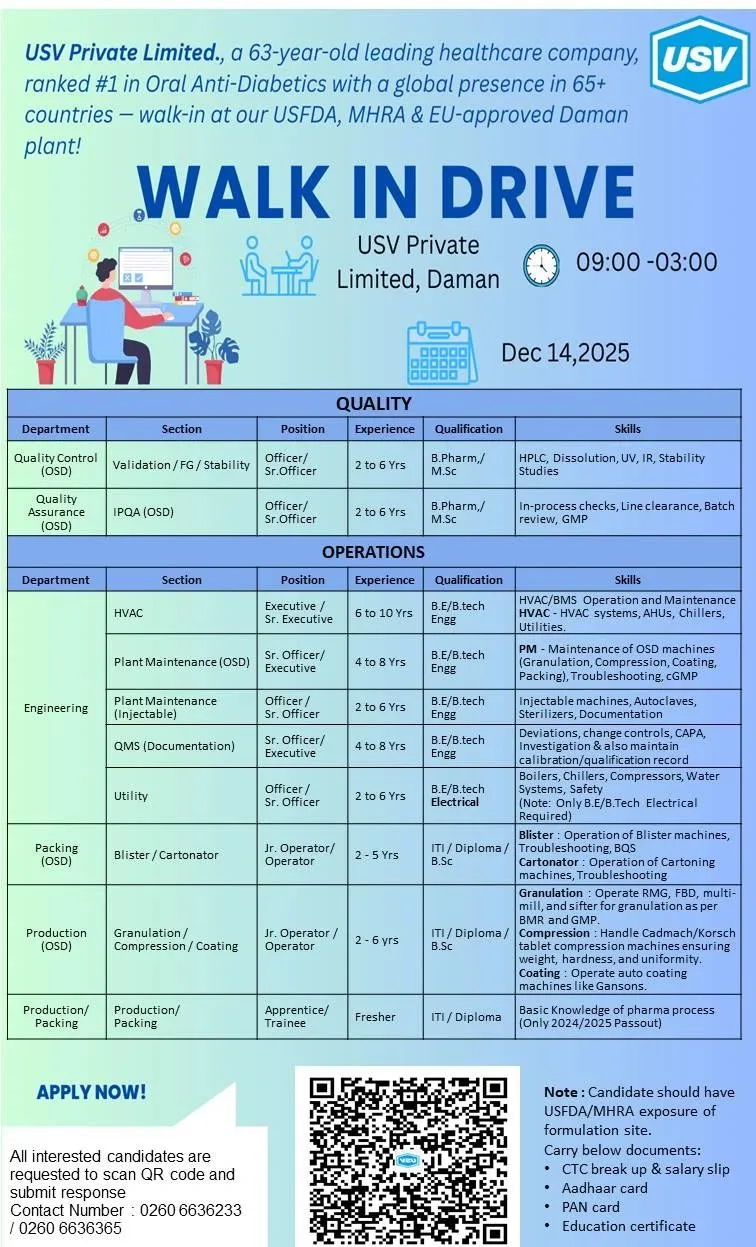
USV Private Limited, a company with a 63-year heritage and a leader in Oral Anti-Diabetics, is hosting a significant walk-in drive for its USFDA, MHRA & EU-approved Daman facility. This event offers diverse opportunities across Quality and Operations for both experienced professionals and recent pass-outs.
Event Details
-
Location: USV Private Limited, Daman.
-
Date: 14th December 2025.
-
Timing: 09:00 AM – 03:00 PM.
-
Registration: Interested candidates must register using the provided Google Form link: https://docs.google.com/forms/d/e/1FAIpQLScjY5anAKDUU74BJVCgowYntfJ9m_XQ-T6q8NwgSoKvtX0y9w/viewform?pli=1.
-
Candidate Profile: Seeking skilled professionals with USFDA/MHRA-regulated formulation plant experience. Freshers (2024/2025 pass outs) can apply for Apprentice/Trainee roles.
Open Roles by Department (OSD Focus)
A. Quality Control (QC)
Roles focus on ensuring product quality through rigorous testing and compliance.
-
Specializations: Validation, Finished Goods (FG), and Stability studies. QC Validation involves qualifying analytical methods and equipment.
B. Quality Assurance (QA)
Maintaining the overall quality system and compliance during manufacturing.
-
Specialization: In-Process Quality Assurance (IPQA), responsible for monitoring manufacturing processes and documentation in real-time.
C. Production (OSD – Oral Solid Dosage)
Hands-on roles in the manufacturing of tablets and capsules.
-
Specializations: Granulation, Compression, and Coating. Expertise in these core OSD unit operations is essential.
D. Packing
Roles dedicated to the final packaging and serialization of OSD products.
-
Specializations: Blister and Cartonator operations. Knowledge of high-speed automatic packing lines, including eye mark recognition systems, is valuable.
E. Engineering
Crucial support roles ensuring facility and equipment compliance and uptime.
-
Specializations: HVAC (Heating, Ventilation, and Air Conditioning), Plant Maintenance, Quality Management System (QMS) documentation related to Engineering, and Utility operations.
F. Apprentice / Trainee
-
Roles: Production and Packing.
-
Target Group: Freshers (2024/2025 pass outs) seeking entry into a regulated pharmaceutical environment.
Documents to Carry
-
Updated Resume.
-
CTC breakup & Salary Slip.
-
Aadhaar & PAN Card.
-
Education Certificates.
2. Anbuva Pharma – Multi Department Walk-In Interviews
Anbuva Pharma is conducting a large-scale recruitment drive over two days for a broad spectrum of roles across its API and Intermediates manufacturing facility. This event is ideal for candidates seeking opportunities in quality functions, core production, and essential support departments.
Event Details
-
Dates: 15th & 16th December 2025 (Monday & Tuesday).
-
Interview Time: 10:00 AM to 4:00 PM.
-
Venue: Shankus Hospitals, B/h Divine Child School, Nr. Shankus Water Park, Ahmedabad-Mehsana Highway, Baliyasan, Mehsana.
-
Contact Info: Mo.: 98246 97733, E-mail: hr@anbuvapharma.com, hetal.pandya@shankus.in.
Open Positions and Eligibility (API/Intermediates Focus)
A. QA & QC & Microbiology
-
Post: Executive/Officer/Chemist.
-
Education: M. Sc / B. Pharm / M. Pharm.
-
Experience: Minimum 1 to 5 years experience in API QA & QC Department. Strong understanding of API regulatory requirements is necessary.
B. Production
-
Post: Executive/Officer/Chemist.
-
Education: B.Sc./M.Sc.
-
Experience: Minimum 1 to 5 years experience in API or Intermediates Manufacturing Department. Familiarity with reactor operations and solvent recovery is key.
C. Engineering
-
Post: Executive/Officer/Engineer.
-
Education: Diploma / BE Mechanical OR Electrical.
-
Experience: Minimum 1 to 5 years experience in API or Engineering Department. Expertise in utility maintenance (boilers, chillers, purified water systems) is often sought.
D. Warehouse
-
Post: Executive/Officer/Engineer.
-
Education: B. Com/ B. Sc.
-
Experience: Minimum 1 to 5 years experience in API Warehouse Department. Knowledge of inventory management, FIFO/FEFO principles, and cGMP storage conditions is required.
E. EHS (Environment, Health, and Safety)
-
Post: Executive/Officer.
-
Education: B.Sc. / B.E. Environment Engineering or Science.
-
Experience: Minimum 1 to 5 years experience in ET Plants (Effluent Treatment Plants). Focus on pollution control, industrial safety, and regulatory reporting.
F. HR Executive
-
Post: HR Executive.
-
Education: BBA/MBA/ Graduation.
-
Experience: Minimum 1 to 3 years experience.
G. Account Executive
-
Post: Account Executive.
-
Education: B. Com/M. Com.
-
Experience: Minimum 1 to 5 years of experience.
3. Axa Parenterals – Multi-Role Walk-In Interview
Axa Parenterals Ltd., along with Heilsa Lifesciences Pvt. Ltd., is conducting an extensive walk-in interview for roles specific to Parenteral (Injectable) and Bottling manufacturing at its Roorkee, Uttarakhand facility. This is a significant opportunity for professionals with experience in sterile manufacturing environments.
Event Details
- Date: 14th December, 2025 (Sunday).
- Timings: 09:00 AM to 02:00 PM.
- Venue: Axa Parenterals Ltd. Puhana chowk, Roorkee, Uttarakhand.
- Contact Info (HR): Kanu Priya (8868033367).
- Target Group: Great Opportunity for Freshers and Experienced candidates.
Open Roles by Department (Parenteral/Injectable Focus)
A. Production/Packing (Sterile Manufacturing)Roles requiring expertise in aseptic filling and specialized equipment.
- Manufacturing Chemist/Officer: Specialization in Ampoule, Vial, LVP (Large Volume Parenterals), and SVP (Small Volume Parenterals).
- Operators: Ampoule Filling/Washing Operator, Vial Filling/Washing/Sealing Operator, Ampoule/ Vial Manufacturing Operator.
- Specialized Roles: Lyophilizer Operator/Technician (handling freeze-drying equipment), CIP/SIP Manufacturing operator (Cleaning/Sterilization In Place), and Packing Supervisor.
- Other Roles: Blister Operator (knowledge of Eye Mark/Rapid Pack Automatic), Filling Operator (3 Pieces).
B. Quality Control (QC)QC roles in a sterile environment demand high precision and instrumental expertise.
- HPLC Executive: Experience with Agilent/Shimadzu instruments is highly valued.
- Officer Stability: Managing stability studies as per ICH guidelines.
- Microbiologist/Trainee Micro: (Male candidates only) Conducting microbial testing, sterility testing, and environmental monitoring.
- Analytical Method Validation Executive: Validating analytical procedures to confirm they are suitable for their intended use.
- QC Chemist.
C. Quality Assurance (QA)Roles focused on managing quality systems and ensuring compliance in sterile operations.
- Assistant Manager-QMS/Executive-QMS: Handling QMS elements like change control, deviations, and CAPA.
- Officer/Executive Validation: Process, equipment, and utility validation.
- Officer IPQA: In-Process Quality Assurance in the sterile and parenteral filling areas.
D. Store (Warehouse)
- Officer/Jr. Officer (RM/PM) / Executive (RM/PM): Handling Raw Material (RM) and Packaging Material (PM) with specific experience in Parenteral storage conditions.
E. Bottle Pack
- Operator (BFS & FFS): Expertise in Blow-Fill-Seal (BFS) and Form-Fill-Seal (FFS) technologies.
- Sr. Engineer Bottle Pack.
Document Required
-
Bring your Updated Resume.
4. BioMatrix Healthcare – Walk-In Interview for QC / Production
BioMatrix Healthcare is looking for motivated professionals for its Oral Solid Dosage (OSD) manufacturing facility in Ahmedabad, focusing on key roles in Quality Control and Production.
Event Details
-
Date: 14th December 2025 (Sunday).
-
Time: 09:00 AM – 12:00 PM.
-
Venue: Corporate Office, 502-B, Times Square Grand, Sindhu bhavan Road, Thaltej, Ahmedabad-380059.
-
Contact Info: 63574 10551, 63589 70525, 97259 01031, 92288 93058.
-
Email for CV Submission: career@biomatrixhealthcare.com, hr@biomatrixhealthcare.com.
Open Positions and Job Descriptions
A. Quality Control (QC)
-
Designation: Officer to Executive.
-
Experience: 2 to 8 Years.
-
Qualification: B.Sc, M.Sc, B.Pharm.
-
Job Description: Strong experience in HPLC operation, performing stability studies, conducting analytical method validation (AMV), and maintaining comprehensive QC documentation in strict compliance with cGMP and regulatory standards.
B. Production (OSD – Oral Solid Dosage)
-
Designation: Officer to Executive.
-
Experience: 2 to 8 Years.
-
Qualification: B.Pharm, M.Pharm.
-
Job Description: Competent and motivated candidates with sound experience in Oral Solid Dosage manufacturing processes.
C. Production Operator (OSD)
-
Designation: Operator.
-
Experience: 2 to 6 Years.
-
Qualification: ITI.
-
Job Description: Qualified and experienced Operator with hands-on expertise in Compression and Granulation processes within Oral Solid Dosage (OSD) manufacturing.
Documents Required
-
Carry your updated CV.
-
Passport size photo.
-
Last three months of salary slip.
-
Latest academic documents.
5. Parexel – Walk In Drive for Regulatory Affairs
Parexel, a leading Clinical Research Organization (CRO) and consulting firm, is hosting a specialized walk-in drive for experienced professionals in the critical field of Regulatory Affairs (RA). These roles are essential for guiding pharmaceutical products through complex global regulatory pathways.
Event Details
-
Date: 20th December, 2025.
-
Time: 10:00 AM Onwards.
-
Venue: Arliga Ecoworld, Building No. 5B, 3rd floor, Sarjapur Marathali, Outer Rind Road, Devarabeesanahalli Village, Varthur, Hobli, Bangalore East Taluk, Bengaluru – 560103.
Open Roles and Key Expertise (2 to 10 Years)
A. Regulatory Intelligence and Regulatory Affiliate Roles
-
Open Roles: Regulatory Intelligence Specialist, Regulatory Affiliate Specialist.
-
Experience: 2–6 years.
-
Key Skills (Regulatory Intelligence): Staying updated on evolving local and regional regulatory requirements, interpreting guidelines, communicating insights, and participating in regulatory forums.
-
Key Skills (Regulatory Affiliate): Reviewing and submitting dossiers, negotiating with Health Authorities for approvals, and collaborating with local cross-functional teams (Medical, Marketing) to support market access and assess regulatory impact.
B. Core Regulatory Affairs Roles (Biologics, Labelling, CMC)
-
Open Roles: Regulatory Affairs Consultant, Senior Regulatory Affairs Associate, Regulatory Affairs Associate.
-
Experience: 2–10 years.
-
Key Skills:
-
Experience in Biologics: Handling lifecycle management, renewals, and global submissions (US, EU, Japan, Canada, Switzerland, Australia) for biologics, vaccines, and monoclonal antibodies.
-
Labelling Expertise: Knowledge of country-specific regulations, CCDS (Company Core Data Sheet)/USPI (US Prescribing Information), SPL (Structured Product Labeling), packaging components, and managing post-approval label updates.
-
Global Frameworks: Strong understanding of global regulatory frameworks and CMC (Chemistry, Manufacturing, and Controls) requirements.
-
Application Links
Candidates are strongly encouraged to check the complete job descriptions before attending the drive or apply online:
-
Senior Regulatory Affairs Associate (Regulatory Intelligence/Affiliate): https://jobs.parexel.com/en/job/bengaluru/senior-regulatory-affairs-associate/877/89330191680
-
Regulatory Affairs Consultant (Biologics/Small Molecules): https://jobs.parexel.com/en/job/bengaluru/regulatory-affairs-consultant-biologics-small-molecules/877/86497930864
-
Senior Regulatory Affairs Associate (Biologics/Small Molecules): https://jobs.parexel.com/en/job/bengaluru/senior-regulatory-affairs-associate-biologics-small-molecules/877/86497930800
-
Senior Regulatory Affairs Associate (Labelling): https://jobs.parexel.com/en/job/bengaluru/senior-regulatory-affairs-associate-labelling/877/86582077104