Attend FDC Limited’s Walk-In Interview from 16–21 June 2025 in Baddi for Production, Quality Assurance, and QC Officer roles. Great opportunity for B.Pharm, M.Pharm, and M.Sc graduates with 0–3 years of pharma experience. USFDA-approved facility. Apply now!
FDC Limited Walk-In Interview June 2025: Hiring for Production, QA & QC Officer Roles in Baddi
Are you a recent B.Pharm, M.Pharm, or M.Sc (Chemistry) graduate looking to start or grow your career in the pharmaceutical industry? FDC Limited, one of India’s top pharmaceutical companies, invites eligible candidates to attend its walk-in interview drive from 16th to 21st June 2025 at its state-of-the-art manufacturing unit in Baddi, Himachal Pradesh.
This walk-in opportunity is open for Production Officers, Quality Control (QC) Officers, and Quality Assurance (QA) Officers with 0–3 years of experience in pharma manufacturing, analytical testing, and regulatory compliance.
🏢 About FDC Limited
Established in 1936, FDC Limited is a pioneering Indian pharmaceutical company with a strong global footprint. It is renowned for its leadership in:
-
Oral Rehydration Salts (ORS)
-
Ophthalmics
-
Active Pharmaceutical Ingredients (APIs)
The company is certified by USFDA, UK-MHRA, and UAE Health Authorities, and it exports to over 50 countries. With more than 5,000 employees and 300+ products, FDC continues to lead innovations in formulations, generics, and consumer healthcare.
🔍 Why Choose FDC Limited?
Here’s why job seekers prefer FDC Limited for pharma careers:
-
✅ Job Security Rating: 3.8/5 (AmbitionBox)
-
✅ Learning & Development: 3.7/5
-
✅ Global Compliance: USFDA & MHRA-approved facilities
-
✅ Career Stability: 5,000+ employee workforce
-
✅ Additional Benefits: Subsidized canteen, PF, transport.
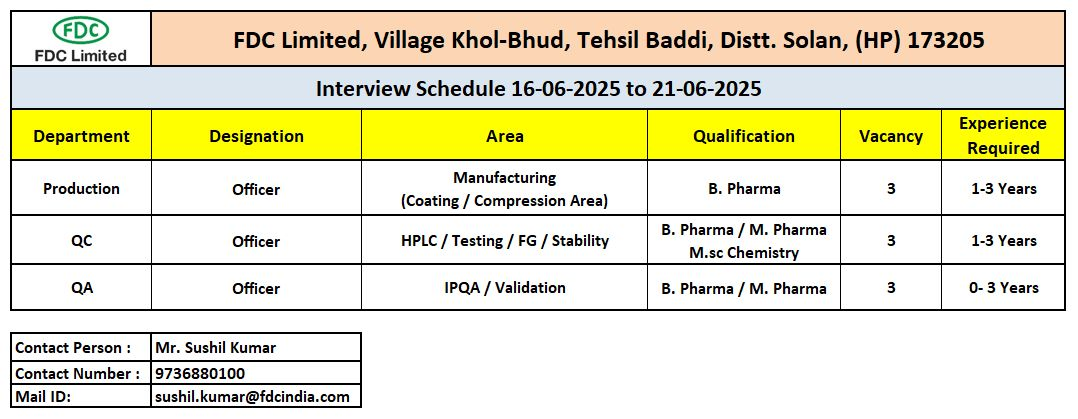
📌 Open Positions at Baddi Pharma Hub
FDC Limited is hiring for multiple officer-level roles in Production, Quality Control (QC), and Quality Assurance (QA) at its Khol-Bhud facility in Baddi, Himachal Pradesh.
🧪 1. Production – Officer
-
Qualification: B.Pharm
-
Experience: 1–3 years
-
Area: Coating & Compression
-
Location: Baddi, HP
-
Key Responsibilities:
-
Operate and calibrate coating and compression machines
-
Prepare and maintain BMR/BPR documentation
-
Ensure adherence to cGMP guidelines
-
Prepare for USFDA/MHRA audits
-
-
Preferred Skills:
-
Hands-on with Cadmach, pan coaters
-
Familiarity with cGMP and safety SOPs
-
Willingness to work in rotational shifts
-
-
Vacancies: 3
-
Note: Male candidates preferred due to shift timings
🔬 2. Quality Control (QC) – Officer
-
Qualification: B.Pharm / M.Pharm / M.Sc (Chemistry)
-
Experience: 1–3 years
-
Area: HPLC, FG Testing, Stability
-
Location: Baddi, HP
-
Key Responsibilities:
-
Perform chemical analysis using HPLC, UV, wet chemistry
-
Conduct method validation, instrument calibration
-
Maintain GLP and cGMP compliance
-
Assist in regulatory audit documentation
-
-
Preferred Skills:
-
Strong command on Empower, Shimadzu
-
Deep knowledge of ICH guidelines
-
Strong documentation accuracy
-
-
Vacancies: 3
-
Note: General shift with some flexibility
🧾 3. Quality Assurance (QA) – Officer
-
Qualification: B.Pharm / M.Pharm
-
Experience: 0–3 years (Freshers eligible)
-
Area: IPQA, Validation
-
Location: Baddi, HP
-
Key Responsibilities:
-
Perform IPQA checks during manufacturing
-
Participate in validation of processes/equipment
-
Document and track QMS deviations, CAPAs
-
Support audit readiness activities
-
-
Preferred Skills:
-
Knowledge of IPQA, cleaning validation, QMS
-
Awareness of USFDA/MHRA compliance
-
Freshers with understanding of cGMP welcome
-
-
Vacancies: 3
-
Note: General shift, freshers preferred
📅 Walk-In Interview Schedule
-
Dates: 16th June – 21st June 2025 (Monday to Saturday)
-
Time: 9:00 AM onwards (arrive early)
-
Venue:
FDC Limited,
Village Khol-Bhud,
Tehsil Baddi, District Solan,
Himachal Pradesh – 173205
📞 Contact for Queries
-
HR Contact: Mr. Sushil Kumar
-
Phone: +91 9736880100 (9:00 AM – 5:30 PM)
-
Email: sushil.kumar@fdcindia.com
-
Website: www.fdcindia.com
🧾 Documents to Carry
-
Updated resume with passport photo
-
Academic certificates (B.Pharm/M.Pharm/M.Sc)
-
Aadhaar & PAN (photocopies)
-
Payslips (last 3 months) / Increment letter (if applicable)
-
Relieving or Experience letters (if applicable).
✅ How to Apply
Interested candidates should attend the walk-in interview with all required documents. If unable to attend in person, email your resume to sushil.kumar@fdcindia.com with subject:Application for [Role] – Baddi – June 2025.
🌐 Why Baddi?
Baddi is one of India’s fastest-growing pharmaceutical manufacturing hubs, located just 40 km from Chandigarh. The area offers:
-
✅ Affordable living costs
-
✅ Employment-rich pharma ecosystem
-
✅ Easy highway access (NH-21A)
-
❗ Public transport is limited; own vehicle preferred.
⚠️ Important Guidelines
-
✅ Freshers eligible only for QA roles; others require 1–3 years of pharma experience
-
✅ Male candidates preferred for production (rotational shifts)
-
✅ Preference given to Himachali candidates
-
❌ No application fees required
-
❌ Avoid job scams – contact only through official channels.
🚀 Don’t Miss This Opportunity!
Kickstart your career with FDC Limited, a global pharmaceutical leader. Whether you’re a fresher or have experience in manufacturing, QA, or QC – this is your chance to work with a USFDA-accredited pharma company.
Mark your calendar for 16th to 21st June 2025 and visit the FDC Baddi facility for the walk-in interview.
Bring your documents, showcase your skills, and step into a promising future in pharma manufacturing!