Dishman Carbogen Amcis is hiring for QA, ADL, R&D, and Engineering roles in Ahmedabad. Attend the walk-in on June 29, 2025. USFDA-approved API manufacturing careers.
💼 Dishman Carbogen Amcis Walk-In Drive 2025: QA, R&D, ADL & Engineering Jobs in Ahmedabad
Are you looking to build a career with a USFDA-approved pharma company offering global exposure and career advancement? Here’s your opportunity to join Dishman Carbogen Amcis, a globally recognized leader in API manufacturing, contract research, and drug development services.
Dishman Carbogen Amcis is conducting a mega walk-in interview for Quality Assurance, Analytical Development (ADL), Research & Development, and Engineering positions at their Ahmedabad (Bavla/Naroda) facilities.
🏢 About Dishman Carbogen Amcis
Founded in 1983, Dishman Carbogen Amcis has evolved into a world-class CRAMS (Contract Research and Manufacturing Services) organization. With manufacturing units across India, Europe, and China, Dishman offers comprehensive services from early-phase development to commercial manufacturing of APIs and intermediates.
🌐 Key Certifications:
- USFDA, PMDA Japan, MHRA UK approved
- High-containment facilities for oncology APIs
- Expertise in Process R&D, ADL, and GMP validation
- 5000+ employees across global locations
🔗 Visit: www.imdcal.com | Careers
📋 Current Job Openings in Ahmedabad
Dishman is hiring across multiple verticals. Here’s a breakdown of the roles available for professionals with 2 to 15 years of experience.
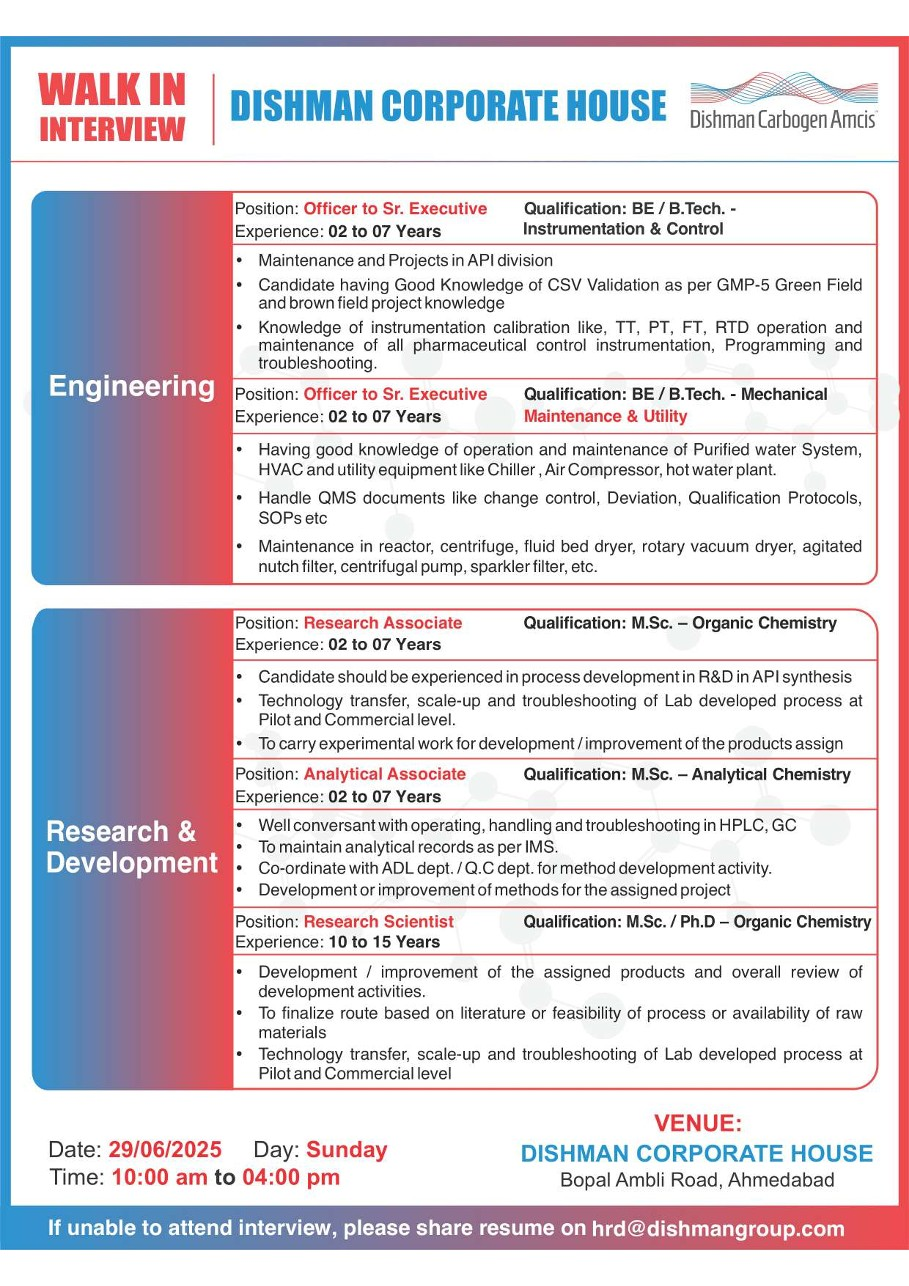
⚙️ Engineering – Instrumentation & Control
Designation: Officer to Sr. Executive
Qualification: B.E./B.Tech (Instrumentation)
Experience: 2–7 years
Key Skills:
- CSV validation (GAMP-5, GMP compliance)
- Instrument calibration: TT, PT, FT, RTD
- PLC programming and troubleshooting
- Greenfield and brownfield project execution
🛠️ Engineering – Maintenance & Utility
Designation: Officer to Sr. Executive
Qualification: B.E./B.Tech (Mechanical)
Experience: 2–7 years
Key Skills:
- Operation/maintenance of purified water, HVAC, chillers
- Handling centrifuges, reactors, fluid bed dryers
- QMS documentation: deviations, SOPs, change control
🔬 Research & Development (API Synthesis)
Research Associate
Qualification: M.Sc. (Organic Chemistry)
Experience: 2–7 years
Research Scientist
Qualification: M.Sc./Ph.D. (Organic Chemistry)
Experience: 10–15 years
Key Responsibilities:
- Process development & scale-up
- Technology transfer from lab to pilot plant
- Troubleshooting and synthetic route optimization
- Literature search, feasibility studies
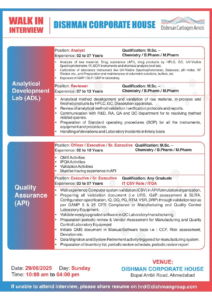
🧪 Analytical Development Laboratory (ADL)
Analytical Associate
Qualification: M.Sc. (Analytical Chemistry)
Experience: 2–7 years
Skills:
- Hands-on with HPLC, GC
- Method development and troubleshooting
- IMS-compliant documentation
Analyst
Qualification: M.Sc. (Chemistry), B.Pharm, M.Pharm
Experience: 2–7 years
Skills:
- Raw material/API/product testing using UV-Vis, KF, IR
- Lab equipment calibration
- Knowledge of GMP, GLP, and GDP
Reviewer
Qualification: M.Sc. (Chemistry), B.Pharm, M.Pharm
Experience: 7–12 years
Skills:
- HPLC/GC method validation
- Dissolution testing, protocol/report review
- SOP drafting, deviation handling
✅ Quality Assurance (API)
Officer/Executive/Sr. Executive
Qualification: M.Sc. (Chemistry), B.Pharm, M.Pharm
Experience: 2–10 years
Responsibilities:
- IPQA, validation protocols, and audit readiness
- QMS documentation and regulatory compliance
- Experience in API batch manufacturing documentation
💻 IT CSV/ITQA – QA Division
Executive/Sr. Executive
Qualification: Any Graduate
Experience: 3–7 years
Skills:
- 21 CFR Part 11, GAMP-5 compliant CSV validation
- URS, IQ, OQ, PQ, RTM, VSR documentation
- Software validation for QC and manufacturing equipment
- QMS handling: risk assessment, deviation closure
🗓️ Walk-In Interview Details
| Date & Time | Venue |
|---|---|
| Sunday, June 29, 2025 (10:00 AM – 4:00 PM) | Dishman Corporate House, Bopal Ambli Road, Ahmedabad, Gujarat |
📎 Documents to Carry:
- Updated Resume
- Educational Certificates
- 3 Months’ Salary Slips
- Bank Statement
- PAN Card / Aadhar Card
- Latest Increment Letter
📩 Can’t Attend? Apply Online
If you’re unable to attend the walk-in interview, send your updated resume to:
📧 hrd@dishmangroup.com
💼 Why Join Dishman Carbogen Amcis?
✅ Rated 3.8/5 for work-life balance by over 200 employees in Ahmedabad
✅ Work in globally certified facilities with USFDA, PMDA, MHRA approvals
✅ Gain cross-functional exposure in R&D, ADL, QA, and Engineering
✅ Strong focus on employee development, training, and safety
✅ International opportunities in Switzerland, UK, and China via Carbogen Amcis global teams
🎯 Ideal Candidate Profile
This hiring drive is ideal for professionals who:
- Are seeking career advancement in API manufacturing and research
- Have 2–15 years of relevant experience in QA, QC, ADL, R&D, or Engineering
- Want to work with USFDA/PMDA/MHRA certified pharma companies
- Aspire to contribute to high-quality global drug development projects.
🚀 Shape the Future of Pharma with Dishman
If you’re ready to accelerate your career with a global pharmaceutical innovator, don’t miss this opportunity. Attend the Dishman Carbogen Amcis Walk-In Drive on June 29, 2025, or email your application today.
🔗 Apply Now: careers.imdcal.com
📧 Email: hrd@dishmangroup.com
Transform pharmaceutical innovation with Dishman. See you in Ahmedabad!