Cipla Walk-In Jobs 2025 – Production, QA, Packing, Engineering + Global Calcium, Anthem Biosciences, Avenza & Eugia Pharma Openings
Walk-in job openings in Cipla, Global Calcium, Anthem Biosciences, Avenza Pharmaceuticals, and Eugia Pharma across Production, Packing, QC, QA, Engineering, Warehouse, Regulatory Affairs, and more.
Mega Pharma Walk-In Hiring – Cipla, Global Calcium, Anthem Biosciences, Avenza Pharma & Eugia Pharma Jobs
The pharmaceutical and life sciences industry continues to expand aggressively in 2025, creating significant opportunities for skilled professionals, freshers, and mid-career candidates. Several leading companies—including Cipla Ltd, Global Calcium Pvt. Ltd, Anthem Biosciences, Avenza Pharmaceuticals, and Eugia Pharma Specialties Limited—are conducting mass recruitment through walk-in interviews for multiple technical departments.
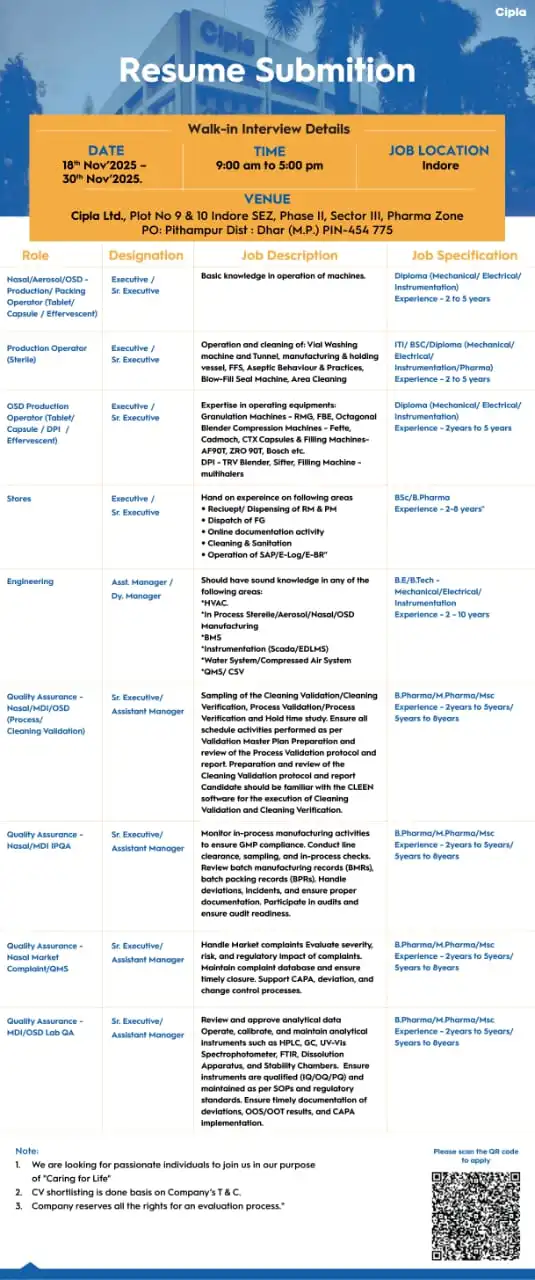
1) Cipla Ltd – Walk-In for Production / Packing / QA / Warehouse / Engineering (Goa | Pithampur)
Cipla remains one of India’s most respected pharmaceutical organizations. Known for their global presence, regulated market exposure, and “Caring for Life” mission, Cipla is hiring experienced professionals with 2–10 years of experience across five major departments.
The walk-in drive is being held at Indore SEZ, Pithampur, for candidates seeking challenging roles in regulated manufacturing environments.
⭐ Cipla Departments Hiring (2–10 Years Experience)
1️⃣ Quality Assurance (QA)
Cipla is looking for experienced QA professionals capable of ensuring compliance across manufacturing processes.
Responsibilities include:
- In-Process Quality Assurance (IPQA) activities
- Review of batch manufacturing records (BMR/BPR)
- Handling of deviations, incidents, CAPA
- Knowledge of cGMP & GDP principles
- Oversight of compliance processes
Candidates with strong knowledge of Quality Systems, QMS documentation, SOP management, and regulatory expectations are ideal.
2️⃣ Production Department
Production functions are central to Cipla’s manufacturing operations.
Key responsibilities:
- Granulation
- Compression
- Coating
- Packaging
- Supervision of production shift activities
- Equipment handling
- Adherence to batch records and SOPs
Professionals experienced with OSD (Oral Solid Dosage), manufacturing operations, and equipment like compression machines, coating pans, granulators, and packing lines are encouraged to attend.
3️⃣ Store / Warehouse
Warehouse roles involve:
- Handling raw materials, packaging materials, and inventory
- SAP knowledge (preferred)
- Warehouse documentation
- Material receipt & issuance
- Maintaining GMP warehouse operations
Understanding of GSP, FIFO, FEFO, and cold storage management is advantageous.
4️⃣ Engineering Department
The engineering division ensures smooth operation of utilities and production systems.
Responsibilities include:
- Utility & equipment maintenance
- Breakdown maintenance
- Preventive maintenance
- Knowledge of HVAC, water system, electrical systems
- Troubleshooting mechanical/electrical issues
Candidates with strong technical expertise in HVAC systems, WFI, compressed air, chilled water, or production machinery maintenance are ideal.
⭐ Cipla Walk-In Interview Details
- Date: 23rd to 30th November 2025
- Time: 9:00 AM – 5:00 PM
- Venue: Cipla Ltd, Plot No. 9 & 10, Indore SEZ Phase II, Sector III, Pharma Zone, Pithampur
⭐ Documents to Carry
- Updated Resume
- Passport-size photo
- Aadhar/PAN
- Educational certificates
- Experience letters
- Salary slips / CTC breakup
- Relevant certifications
Cipla emphasizes their commitment to hiring passionate individuals aligned with their mission of “Caring for Life.”

2) Global Calcium Pvt. Ltd – Hiring for Production / Packing (Freshers & Experienced)
📍 Unit–III, Hosur
📅 25th November 2025 | 9:30 AM – 1:00 PM
Global Calcium is a leading API and mineral-based pharmaceutical manufacturer. The company is hiring Officers and Trainees with 0–5 years of experience.
This opening is perfect for freshers with degrees in:
- M.Sc
- B.Sc
- B.Tech
⭐ Job Roles – Production & Packing
Freshers and experienced candidates will assist with:
- API / Bulk chemical manufacturing
- Batch preparation & record maintenance
- GMP compliance & documentation
- Operating equipment used in production and packing
- Performing checks & assisting in daily plant functions
- Adhering to quality & safety guidelines
- Supporting shift operations
Ideal candidates should be enthusiastic, hardworking, and eager to grow within pharmaceutical manufacturing.
⭐ Global Calcium Walk-In Details
- Date: 25th November 2025
- Time: 9:30 AM – 1:00 PM
- Location: Global Calcium Pvt. Ltd., Unit–III, Hosur
⭐ Documents Required
- Updated Resume
- Passport-size photo
- Educational certificates
- ID proof
- Experience certificates (if applicable)

3) Anthem Biosciences – Walk-In Interviews (Freshers & Experienced)
📍 Unit II – Harohalli, Bangalore
📅 29th November 2025 | 9:00 AM – 1:00 PM
Anthem Biosciences is a leading CRO/CDMO offering chemical synthesis, biologics development, and pre-clinical research services. Their walk-in is specifically for chemical synthesis plant – Unit II.
⭐ Eligible Qualifications
- Diploma (Chemical Engineering)
- B.Sc (Chemistry)
- B.Tech / B.E (Chemical Engineering)
⭐ Experience Required
- 0–1 year in API bulk manufacturing, custom synthesis, or related areas
- Shift-ready candidates are preferred
⭐ Anthem Biosciences Responsibilities
Freshers and experienced candidates may work on:
- Chemical synthesis
- API/intermediate production
- Reaction monitoring
- Batch documentation
- Safety compliance
- Operating chemical processing equipment
- Following SOPs and GMP guidelines
- Handling hazardous chemicals with EHS compliance
⭐ Walk-In Venue
Anthem Biosciences Limited,
KIADB Industrial Area Phase-2,
Harohalli, Near Bannikuppe Village,
Kanakapura Taluk,
Ramanagar District – 562112
4) Avenza Pharmaceuticals – Walk In for Quality Control (Multiple Roles)
📍 Vadodara – Savli
📅 22nd / 23rd / 24th November 2025 | 10 AM – 4 PM
Avenza Pharmaceuticals is conducting a large-scale walk-in for QC professionals with 2–10 years of experience across specialized QC functions.
Freshers are not eligible for these roles.
⭐ QC Open Positions at Avenza Pharma
1. GLP (4–8 yrs)
- Laboratory standard management
- Instrument qualification
- QC preventive maintenance
- Consumables management
2. Analytical Method Validation – AMV (4–7 yrs)
- Method validation/verification
- Knowledge of OSD AMV: Assay, Dissolution, RS, CU/BU
- ICH guideline compliance
3. RM/PM Analyst (2–3 yrs)
- RM classical testing (KF, potentiometry, TLC, titration, etc.)
4. HPLC Analyst – OpenLab (4–7 yrs)
- Hands-on with OpenLab software
- Method creation
- Template preparation
- RS/Assay processing
5. QAMS (5–8 yrs)
- CAPA handling
- STP/specification/TDS review
- SOP management
6. QC Investigation Specialist (4–6 yrs)
- Handling OOS
- OOT
- Lab incident investigations
- Hypothesis testing
- Analyst interview for root cause identification
7. QC Microbiologist (2–5 yrs)
- MLT testing
- Media prep
- EM & water sampling
- Autoclave operation
8. QC Reviewer (8–10 yrs)
- Review of RM/PM/FP testing
- Review of validation & calibration data
- Stability study record verification
⭐ Documents Required
- Updated CV
- Last 3 months salary slips
- Current CTC
Freshers are strictly not considered for QC jobs at Avenza.

5) Eugia Pharma Specialties Limited – Multiple Openings (QA / QC / Production / RA)
Eugia Pharma, a reputed injectable and specialty pharma manufacturer, is hiring professionals with 2–9 years of experience across critical functions.
⭐ Department-Wise Vacancy Details
1) Production (2–9 years)
- Handling sterile/solid dosage manufacturing
- Equipment operation
- Batch documentation
- Adherence to QMS and GMP
2) IPQA (2–5 years)
- In-process checks
- Line clearance
- Documentation review
- Deviation/CAPA support
3) Production – QMS (4–8 years)
- Batch record handling
- QMS documentation
- CAPA, change controls, deviations
4) QA – Market Compliance (4–8 years)
- Compliance for global markets
- Supporting regulatory audits
- Managing GMP documentation
5) MQA – Injectable (4–8 years)
- Injectable manufacturing QA oversight
- Aseptic compliance
- Sterility assurance
6) Production – Softgel (2–6 years)
- Soft gel capsule manufacturing
- Filling/encapsulation processes
7) QC – HPLC, IPFP (2–6 years)
- HPLC testing
- Instrument handling
- FP testing
- Analytical documentation
8) Regulatory Affairs (2–8 years)
- US, EU, Canada, RoW market submissions
- Dossiers, variation filings
- Market compliance & query responses
⭐ Qualification
- M.Pharmacy (mandatory)
⭐ How to Apply
📱 WhatsApp: 83280 53327
Eugia seeks energetic candidates who want to grow in a regulated pharmaceutical environment.