Top Clinical Research jobs. Hiring for CRA, Medical Writer, and Data Management roles at Thermo Fisher, Accenture, Sun Pharma, and Synergen Bio. Apply now!
The clinical research landscape in India is undergoing a massive transformation as we head into 2025. With a surge in global outsourcing and the rapid adoption of digital trial technologies, major industry leaders like Thermo Fisher Scientific, Accenture, Sun Pharma, Synergen Bio, and Cliantha Research have opened multiple high-impact positions. These roles span across Clinical Research Associates (CRA), Medical Writers, Regulatory Affairs Executives, and Clinical Data Management (CDM), offering a unique blend of on-site and 100% remote work opportunities for life science graduates, pharmacists, and medical professionals.

1 Synergen Bio Clinical Research Associate CRA Recruitment
Synergen Bio Pvt. Ltd. is currently expanding its clinical operations team in Pune. The company is seeking Clinical Research Associates (CRA) to support their Bioavailability and Bioequivalence (BA/BE) studies. This role is ideal for candidates who prefer a hands-on, on-site environment and possess a deep understanding of study execution and regulatory compliance.
Job Overview and Core Requirements
The CRA position at Synergen Bio is open to candidates with 1 to 2+ years of experience. The role requires a strong academic background, specifically an M.Sc. or B.Pharm degree. Based in the Shivajinagar and Wakadewadi areas of Pune, this on-site role focuses heavily on the meticulous execution of BA/BE studies according to Standard Operating Procedures (SOPs) and global regulatory guidelines such as ICH-GCP and DCGI.
Key Responsibilities and Skill Set
-
Study Execution: Overseeing the day-to-day operations of BA/BE studies to ensure they meet quality and timeline benchmarks.
-
Documentation: Managing the Trial Master File (TMF), reviewing Case Report Forms (CRFs), and ensuring the accuracy of raw data.
-
Participant Safety: Handling informed consent processes and screening activities with a high level of ethics and professionalism.
-
Stakeholder Coordination: Acting as a bridge between bioanalytical teams and clinical investigators, providing regular updates on study progress.
Why Join Synergen Bio?
Synergen Bio offers a collaborative work culture where quality is the top priority. Employees gain significant exposure to global regulatory standards, making it an excellent platform for professionals looking to transition into international clinical research roles. To apply, candidates can email their CV to careers@synergenbio.com with the subject line “CRA Application.”

2 Cliantha Research Medical Writer Careers
Cliantha Research is a leading global Contract Research Organization (CRO) that is currently hiring experienced Medical Writers for its Ahmedabad corporate office. This role is a critical pillar of the clinical trial process, ensuring that all scientific and regulatory documents meet the highest standards of clarity and compliance.
Eligibility and Role Definition
The Medical Writer position at Cliantha is designed for B.Pharm graduates with 2 to 4 years of specialized experience in medical writing. This is an on-site role that requires a blend of scientific expertise and advanced writing skills. Candidates must be well-versed in ICH-GCP guidelines and have a proven track record of preparing regulatory documents that withstand international scrutiny.
Primary Duties of a Medical Writer
-
Protocol Development: Designing comprehensive clinical study protocols that outline the objectives, design, methodology, and statistical considerations of a trial.
-
Regulatory Documentation: Creating Informed Consent Documents (ICDs), Case Report Forms (CRFs), and Clinical Study Reports (CSRs).
-
Collaboration: Consulting with Principal Investigators, Biostatisticians, and Analytical Investigators to ensure data is interpreted and presented accurately.
-
Compliance Management: Ensuring every document aligns with specific country-level regulatory requirements.
Applying to Cliantha Research
Interested professionals should use the official Cliantha careers portal to submit their applications. The specific job code for this requirement is Med–2025-11-21-971. Joining Cliantha provides long-term stability and the opportunity to work on diverse global clinical research projects.
How to Apply
Interested candidates can apply through the official Cliantha Research careers portal or job portals listing this position (Job Code/Reference: Med–2025-11-21-971).
3 Thermo Fisher Scientific 100% Remote Clinical Data Management CDM Drive
In one of the most significant recruitment drives of 2025, Thermo Fisher Scientific is expanding its Functional Service Provider (FSP) team in India. They are offering multiple roles in Clinical Data Management (CDM) that are 100% fully remote, providing unprecedented flexibility for professionals across India.
A Career Ladder in CDM
Thermo Fisher has openings ranging from entry-level positions to senior management roles. The experience requirements vary from 1 year to over 10 years:
-
Clinical Data Associate I (1-1.6 Years): Focuses on basic data cleaning and reconciliation using EDC systems like RAVE or Veeva.
-
Coding Specialist (1-1.6 Years): Requires expertise in medical terminology and coding dictionaries such as WHODD and MedDRA.
-
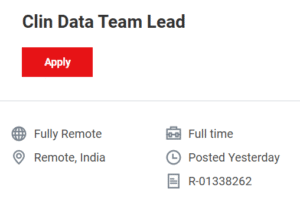
Clinical Data Team Lead (4-5.6 Years): Responsible for end-to-end study management and database lock procedures.
-
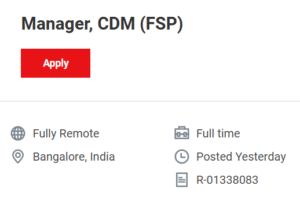
Manager, CDM (10+ Years): Involves high-level team management, global resourcing, and client liaison.
The Remote Advantage
These roles are part of a mature delivery model that utilizes AI-enabled workflows and modern electronic data capture (EDC) systems. Working remotely for a global giant like Thermo Fisher allows professionals to gain international experience while maintaining a healthy work-life balance. Candidates are encouraged to apply directly through the Thermo Fisher Workday portal using the specific requisition IDs provided in the job listings.
How to Apply
Apply directly through the official Thermo Fisher Scientific careers portal:
🔗APPLY HERE for Clinical Data Associate I (R-01338259)
https://jobs.thermofisher.com/global/en/job/R-01338259/Clin-Data-Assoc-I
🔗APPLY HERE Clinical Data Associate II (R-01338260)
https://jobs.thermofisher.com/global/en/job/R-01338260/Clin-Data-Assoc-II
🔗APPLY HERE for Coding Specialist (R-01338263)
https://jobs.thermofisher.com/global/en/job/R-01338263/Coding-Specialist
🔗APPLY HERE for Assoc Clin Data Team lead (R-01338261)
https://jobs.thermofisher.com/global/en/job/R-01338261/Assoc-Clin-Data-Team-Lead
🔗APPLY HERE for Clin Data Team Lead (R-01338262)
https://jobs.thermofisher.com/global/en/job/R-01338262/Clin-Data-Team-Lead
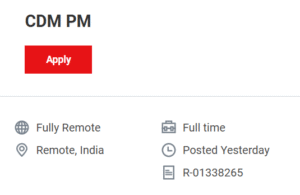
🔗APPLY HERE for CDM PM (R-01338265)
https://jobs.thermofisher.com/global/en/job/R-01338265/CDM-PM
🔗APPLY HERE for Manager, CDM (FSP) (R-01338083)
https://jobs.thermofisher.com/global/en/job/R-01338083/Manager-CDM-FSP
4 Accenture Clinical Data Services Medical Monitoring
Accenture is looking for Clinical Data Services Associates for its Life Sciences R&D vertical in Bengaluru. This role is unique as it focuses on medical monitoring and trial oversight, making it an ideal fit for B.Tech (Biotech), B.Pharm, or M.Pharm graduates with 1 to 3 years of experience.
Role and Responsibilities
The primary focus of this position is ensuring the safety and integrity of clinical trials through rigorous medical monitoring. Associates are responsible for:
-
Trial Oversight: Providing medical expertise to monitor patient safety and address any clinical concerns during the trial.
-
Protocol Validation: Reviewing and validating protocol specifications to ensure they meet regulatory and scientific requirements.
-
Problem Solving: Using predefined guidelines to solve routine data issues and ensuring data collection follows established SOPs.
Accenture provides a highly innovative and inclusive work culture. This role offers the chance to work at the intersection of life sciences and technology, helping to drive better healthcare outcomes globally. The job requisition ID for this role is AIOC-S01619664.
How to Apply
Apply online through the official Accenture careers portal.
🔗APPLY HERE https://www.accenture.com/in-en/careers/jobdetails?id=AIOC-S01619664_en&title=Clinical+Data+Svs+Associate
5 Sun Pharma Regulatory Affairs and Medical Writing Roles
Sun Pharmaceutical Industries Ltd, India’s largest pharma company, is hiring for key positions in Baroda and Mumbai. These roles are geared toward experienced professionals who want to work with a global leader in generic and specialty medicines.
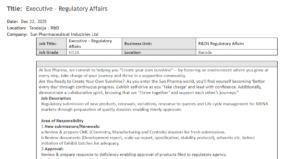
Executive – Regulatory Affairs (Baroda)
This role (Grade G12A) focuses on the MENA market (Middle East and North Africa). With 1 to 4 years of experience, M.Sc or M.Pharm graduates will handle CMC dossiers, deficiency responses, and lifecycle management for site changes and harmonization.
Medical Writer (Mumbai)
Sun Pharma is looking for MBBS professionals with 3 to 5 years of experience in clinical trials. This role involves developing protocols, Investigator Brochures (IB), and Clinical Study Reports (CSR) for both interventional and non-interventional studies. Candidates must have publication experience in indexed journals and a deep understanding of CDSCO and ICH-GCP guidelines.
How to Apply
Apply directly through the official Sun Pharma careers portal at https://careers.sunpharma.com/. Search for “Executive – Regulatory Affairs” (Baroda) or “Medical Writer” (Mumbai) positions and submit your updated resume.
🔗APPLY HERE for Regulatory Affairs https://careers.sunpharma.com/job/Baroda-Executive-Regulatory-Affairs/46936944/
🔗APPLY HERE for Medical Writer https://careers.sunpharma.com/job/Mumbai-Medical-Writer-%28Clinical-Trials%29/48045244/
Strategic Career Growth in Clinical Research 2025
The current job market highlights three major trends that candidates should leverage to build a successful career:
1 The Shift to Remote and Hybrid Models
The drive by Thermo Fisher illustrates that CDM and Medical Writing are no longer bound to physical offices. Developing proficiency in remote collaboration tools and cloud-based EDC systems is now a mandatory skill for modern researchers.
2 Regulatory Complexity and Compliance
Whether it is Synergen Bio’s focus on BA/BE studies or Sun Pharma’s MENA market submissions, there is a rising demand for professionals who understand “country-specific variations.” Specializing in a particular geography (like the USFDA, EMA, or GCC) can significantly increase your market value.
3 Technological Integration
Accenture and Thermo Fisher are increasingly integrating AI and data analytics into their clinical services. Professionals who upskill in data science, medical coding (MedDRA), and automated query management will find themselves at a distinct advantage during the hiring process.
Hiring Locations and Roles
| Company | Key Role | Location | Work Mode |
| Synergen Bio | Clinical Research Associate | Pune | On-site |
| Cliantha Research | Medical Writer | Ahmedabad | On-site |
| Thermo Fisher | CDM (Various Levels) | Pan India | 100% Remote |
| Accenture | Data Services Associate | Bengaluru | On-site |
| Sun Pharma | Regulatory / Medical Writer | Baroda / Mumbai | On-site |
How to Optimize Your Application for These Roles
To stand out in these competitive recruitment drives, candidates should tailor their resumes to the specific “mandatory requirements” mentioned by the companies.
-
For CRA Roles: Highlight your knowledge of SOPs and your experience with informed consent processes and TMF management.
-
For CDM Roles: Explicitly mention your experience with RAVE or Veeva EDC. Mentioning specific dictionaries like WHODD will help you pass ATS filters.
-
For Medical Writing: Provide a list of document types you have authored (e.g., protocols, CSRs, or manuscripts) and mention your familiarity with various reporting guidelines like CONSORT or STROBE.
-
For Regulatory Affairs: Emphasize your experience with deficiency responses and lifecycle management.