Join Endo India Par Formulations for a walk-in interview on 3rd August 2025 in Hyderabad. Open roles in microbiology, laboratory operations, and QMS for experienced professionals. Positions based in Indore. Apply now for a career with innovation and purpose!
Endo India Par Formulations – Walk-In Interview for Microbiology & QMS Roles on 3rd August 2025
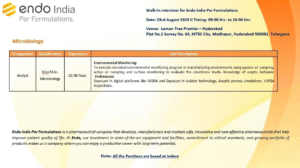
Endo India Par Formulations, a trusted name in pharmaceutical innovation and manufacturing, is conducting a walk-in interview drive on August 3, 2025, in Hyderabad. We are hiring experienced professionals in microbiology, quality management systems (QMS), and laboratory operations. If you are passionate about contributing to life-changing pharmaceutical development, join us and explore long-term career potential with a global leader.
📍 Walk-In Interview Details
- Date: Sunday, August 3, 2025
- Time: 9:00 AM to 4:00 PM
- Venue: Lemon Tree Premier, HITEC City, Madhapur, Hyderabad – 500081
- Note: All roles are based in Indore, Madhya Pradesh
👩🔬 Available Job Openings
| Designation | Qualification | Experience | Location |
|---|---|---|---|
| Sr. Analyst | B.Sc./M.Sc. Microbiology | 4–10 Years | Indore |
| Analyst | B.Sc./M.Sc. Microbiology | 2–6 Years | Indore |
🧪 Key Roles & Responsibilities
1. Senior Analyst – Laboratory Operations
- Sterility testing, BET (Gel Clot/KTA), MLT, and media management
- USFDA compliance and equipment calibration
- Experience with isolators and digital platforms (LIMS, MODA)
2. Senior Analyst – Document Review / QMS
- Review microbiology data, SOPs, validations
- Handle TrackWise, deviation management, CAPA
- Expertise in regulatory audit readiness
3. Analyst – Environmental Monitoring
- Execute cleanroom microbial monitoring
- Aseptic behavior knowledge and isolator exposure
- Hands-on with digital tools like MODA
🎯 Why Work at Endo India Par Formulations?
- ✅ Innovation-Driven: Leverage cutting-edge technologies and global projects
- ✅ Career Progression: Defined growth paths and professional development
- ✅ Global Standards: USFDA-compliant operations and ethical environment
- ✅ Impactful Role: Contribute directly to patient safety and therapeutic innovation
📌 What to Bring for the Interview
- Updated Resume & Academic Documents
- Certificates of Experience and Special Training
- Knowledge of USFDA, GMP, and pharma regulations
- Readiness to relocate to Indore, MP
🔗 Official Links
Endo India Par Formulations is your opportunity to be a part of a forward-thinking organization where science meets purpose. Don’t miss this chance to elevate your pharma career—walk in on 3rd August 2025 and be part of a healthier tomorrow.