1. Gowrie Research Walk-In Interview – R&D & Regulatory Roles
Location: Vadodara, Gujarat
Dates: September 22–26, 2025
Time: 11:00 AM – 8:00 PM
Venue: Gowrie Research Pvt. Ltd., BP Estate, N.H. No.8, Nr. Prakruti Resort, Chhani, Vadodara
Job Opportunities at Gowrie Research
Formulation & Development (F&D)
-
Qualification: B.Pharm / M.Pharm
-
Experience: 1–5 years
-
Roles: Officer / Sr. Officer / Executive
-
Responsibilities: Conduct formulation experiments, optimize processes, ensure GMP compliance, prepare documentation.
Regulatory Affairs (EU/UK Market)
-
Qualification: B.Pharm / M.Pharm
-
Experience: 2–4 years
-
Roles: Officer / Sr. Officer
-
Responsibilities: Prepare dossiers for EU/UK approvals, manage regulatory changes, maintain compliance.
Business Development & Product Development
-
Qualification: B.Pharm / M.Pharm
-
Experience: 3–7 years
-
Roles: Executive / Sr. Executive
-
Responsibilities: Identify market opportunities, manage product lifecycle, support tech transfer.
Regulatory Affairs (PLPI – Trainee)
-
Qualification: B.Sc / M.Sc / B.Pharm
-
Experience: 0–1 years (Freshers welcome)
-
Role: Trainee
-
Responsibilities: Assist in PLPI filings, learn regulatory processes, support compliance.
Why Join Gowrie Research?
-
Competitive pay packages
-
Training in R&D and regulatory processes
-
Global exposure through EU/UK submissions
-
Innovative and collaborative work culture
How to Apply: Attend walk-in or email CV to jobs@bnsgroup.co.uk / darshan.joshi@bnsthamelabs.com.
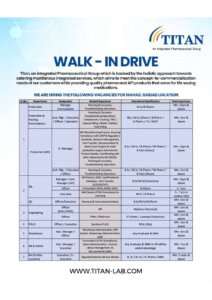
2. Titan Laboratories Walk-In Drive – API, Formulation, QA & More
Locations: Mahad (Raigad) & Turbhe (Navi Mumbai)
Dates: September 21, 22–30, 28, 2025
Venues:
-
Hotel Mirage, Roha (Sept 21)
-
Hotel Reemz, Chiplun (Sept 28)
-
Titan Pharmaplus, Mahad (Sept 22–30)
-
Titan Laboratories, Turbhe (Sept 22–30)
Key Job Departments
Production (Formulation & API)
-
Roles: Manager, Asst. Manager, Executive, Officer, Operators
-
Qualification: B.Sc/M.Sc (Chemistry), B.Pharm, M.Pharm, B.Tech (Chem)
-
Experience: 4–15+ years
Quality Assurance (QA – API & Formulation)
-
Roles: Manager, Officer, Executive
-
Qualification: B.Sc/M.Sc (Chem), B.Pharm
-
Experience: 3–15 years
Engineering
-
Roles: Officer, Electrician, Fitter
-
Qualification: Diploma / ITI
-
Experience: 5+ years
R&D, Warehouse, HR, Regulatory Affairs
-
Openings across Mahad & Turbhe with focus on compliance and Asia regulatory submissions.
Why Titan Laboratories?
-
Exposure to both API & formulation plants
-
Structured career growth
-
Opportunities in multiple functional areas
-
Focus on regulatory-driven manufacturing
How to Apply: Email resume to sandesh.k@titanpharma.com or hrd1@titanpharmaplus.in.
3. Reliance Life Sciences – Young Professional Program (YPP)
Date: September 21, 2025
Time: 9:00 AM – 12:00 Noon
Venue: Reliance Retail Ltd., Tower A, IBC Knowledge Park, Bengaluru
Work Location: Navi Mumbai
About the Program
The Young Professional Program is a one-year structured training program for freshers in biotechnology and biosimilars.
-
Eligibility: M.Sc (Biotech, Microbiology, Biochemistry), B.E/B.Tech/M.Tech (Biotechnology)
-
Experience: Freshers welcome
-
Focus Areas: GMP training, plasma proteins, biosimilars, production & quality systems
Benefits of YPP
-
Paid training with competitive compensation
-
Retention bonus
-
Career advancement opportunities
-
Mentorship by industry experts
How to Apply: Attend the walk-in or visit www.rellife.com.
4. Immacule Lifesciences – 69+ Openings in Oncology Injectables
Date: September 21, 2025
Time: 9:30 AM – 2:00 PM
Venue: Immacule Lifesciences Pvt Ltd, New Nalagarh, Himachal Pradesh
Available Positions
Production – 40 Openings
-
Officers/Executives/Operators
-
Qualification: ITI/Diploma/B.Sc/B.Pharm/M.Pharm
-
Experience: 3–9 years
Engineering – 20 Openings
-
Roles in breakdown & preventive maintenance
-
Qualification: Diploma/B.Tech (Electrical & Instrumentation)
Quality Assurance (Validation) – 10 Openings
-
Roles: Officer/Executive/Sr. Executive
-
Qualification: B.Pharm/M.Pharm
Why Join Immacule?
-
Specialization in oncology injectables
-
Experience in USFDA-approved facility
-
Growth in regulatory-driven environment
5. JAMP India Pharmaceuticals – Regulatory Affairs (Biosimilars)
Location: Ahmedabad, Gujarat
Role: Officer/Sr. Officer – Regulatory Affairs (Biosimilars)
-
Qualification: B.Pharm/M.Pharm
-
Experience: 1–3 years
-
Skills: Biosimilar submissions, GMP, GDP compliance
Benefits
-
International exposure to biosimilar regulations
-
Growth in regulatory affairs careers
-
Competitive salary + training
How to Apply: Send resume to dkothari@jamppharma.com.
6. Ajanta Pharma Walk-In – QA, QC, Manufacturing & Maintenance
Date: September 28, 2025
Time: 9:00 AM – 4:00 PM
Venue: The Red Maple Mashal, Indore
Work Location: Dahej, Gujarat
Open Positions
Manufacturing & Packing
-
Roles: Sr. Officer, Officer, Operators
-
Skills: OSD operations, compression, coating, capsule filling
Quality Control (QC)
-
Roles: Sr. Officer/Officer
-
Skills: HPLC, UV, dissolution, method validation
Quality Assurance (QA)
-
Roles: Sr. Officer/Officer
-
Skills: IPQA, AQA, technology transfer
Maintenance
-
Roles: Officer, Assistant
-
Qualification: B.E/ITI Electrical
Why Join Ajanta Pharma?
-
Great Place to Work-certified company
-
Strong presence in OSD manufacturing
-
Career advancement and training programs.