Attend major pharmaceutical walk-in interviews on January 9-11, 2026. Top openings at Macleods, Zydus Lifesciences, Ajanta Pharma, Ipca Labs, and Mylan for QC, QA, Production, Engineering, and HR across Gujarat, Bengaluru and Himachal Pradesh.
The pharmaceutical sector in 2026 continues to demonstrate robust growth, creating a high demand for skilled professionals across India’s major industrial hubs. This week, several Tier-1 pharmaceutical companies, including Macleods, Ajanta Pharma, Ipca Laboratories, and Zydus Lifesciences, have announced extensive walk-in recruitment drives. These opportunities span diverse departments such as API Production, Sterile Formulations, Quality Control, Engineering Services, and Human Resources.
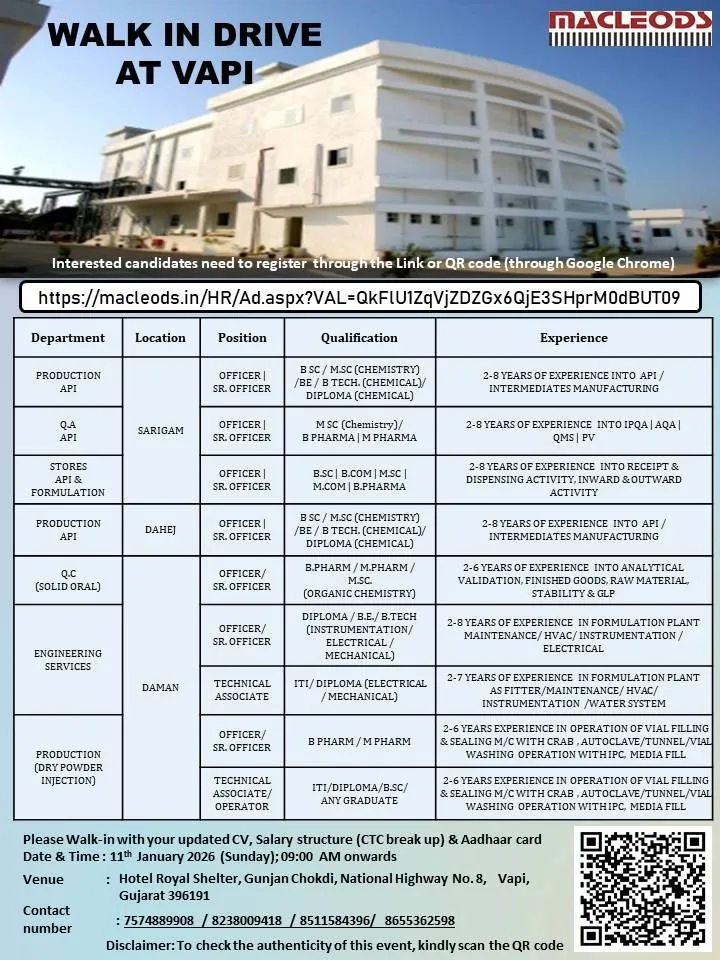
1. Macleods Pharmaceutical – Mega Walk In Drive
Macleods Pharmaceuticals, one of the world’s leading vertically integrated pharmaceutical companies, is conducting a comprehensive recruitment drive for its API and Formulation units located in Sarigam and Dahej.
Event Schedule
-
Date: 11th January 2026 (Sunday)
-
Time: 09:00 AM onwards
-
Venue: Hotel Royal Shelter, Gunjan Chokdi, National Highway No. 8, Vapi, Gujarat – 396191.
Open Departments and Job Roles
-
API Production (Sarigam/Dahej): Officer and Sr. Officer roles for B.Sc/M.Sc Chemistry or Chemical Engineering graduates with 2–8 years of experience in intermediate manufacturing.
-
Quality Assurance – API: Seeking candidates with 2–8 years in IPQA, AQA, or QMS.
-
Quality Control – Solid Oral: Positions for B.Pharm/M.Sc candidates with 2–6 years of experience in stability, analytical validation, and GLP.
-
Engineering Services: Openings for Officers and Technical Associates in HVAC, Instrumentation, and Electrical maintenance.
-
Production – Dry Powder Injection: Focused on sterile manufacturing, seeking those with 2–6 years of experience in vial filling, sealing, and autoclave operations.
-
Stores (API & Formulation): Roles for graduates with 2–8 years in dispensing and material receipt.
2. Ajanta Pharma Ltd – Walk In Interview
Ajanta Pharma, recognized as a “Great Place to Work,” is inviting professionals to join its specialty formulation facility. The company offers excellent amenities, including AC bus facilities for employees from Bharuch and Vadodara.
Event Schedule
-
Date: 11th January 2026 (Sunday)
-
Time: 9:00 AM to 4:00 PM
-
Venue: Hotel Corona, Opp. Railway Station, Bharuch, Gujarat 392001.
Featured Opportunities
-
Manufacturing & Packing: Associate/Operator (ITI/Diploma) and Officer (B.Pharm) roles requiring experience in granulation, coating, and blister packing.
-
Quality Control (QC): Analysts with 2–8 years experience using HPLC and UV spectrophotometers.
-
Maintenance: Openings for B.E Electrical and ITI professionals for plant and packing equipment maintenance.
-
MES (PAS X) Production: A specialized role for Sr. Officers with 2–8 years experience in MBR designing and equipment integration with PAS X systems.
-
Warehouse/Stores: Sr. Officer roles for graduates with 3–10 years in raw material and packing material sections.
3. Zydus Lifesciences – HR Shared Services (Freshers & Experienced)
Zydus Lifesciences is providing a unique entry-point for fresh graduates and early-career professionals in the field of Human Resources. This drive specifically encourages women returning to the workforce.
Event Schedule
-
Date: 9th January 2026
-
Time: 4:00 PM – 5:30 PM
-
Venue: Zydus Corporate Park, Near Nirma University, S.G. Highway, Ahmedabad.
Role Overview
-
Department: Human Resource Shared Services (Contractual)
-
Eligibility: Freshers or those with 0–2 years of experience. Open to all graduates (B.A, B.Sc, B.Com, B.Tech, etc.).
-
Responsibilities: Back-office recruitment support, data management, and assisting HR Business Partners with statutory compliance. Strong proficiency in MS Excel is preferred.
4. Ipca Laboratories – Walk In Interview
Ipca Laboratories is seeking experienced talent for its OSD (Oral Solid Dosage) plant in Silvassa. Note that this drive is strictly for candidates with tablet and capsule experience.
Event Schedule
-
Date: 11 January 2026 (Sunday)
-
Time: 09:00 AM – 4:00 PM
-
Venue: Hotel Woodland, Vapi, National Highway, Vapi, Gujarat 396191.
Department Breakdown
-
Departments: Production, Packing, Quality Control, and QA.
-
Experience Level: Candidates must have exposure to regulated plants (USFDA/MHRA).
-
Important Note: M.Sc candidates will not be considered for the Quality Control department in this specific drive.
5. Mylan Laboratories (Viatris) – Injectable QA Walk In
Mylan Laboratories, now a part of Viatris, is hosting a drive for its specialized injectable manufacturing facility.
Event Schedule
-
Date: 10th January 2026 (Saturday)
-
Time: 10:00 AM to 15:00 PM
-
Venue: Injectables Operations Office, Bommasandra Jigani Link Road, Near APC Circle, Bengaluru – 560105.
Position Details
-
Department: Quality Assurance (IPQA, QMS, Compliance).
-
Qualification: M.Sc Chemistry / B.Pharm / M.Pharm.
-
Experience: 2 to 8 years in sterile/injectable manufacturing environments.
6. PI Industries – Technology Transfer & Pilot Plant Roles
PI Industries is looking for high-caliber chemical professionals for its R&D and manufacturing operations based in Udaipur.
Event Schedule
-
Date: 11 January 2026 (Sunday)
-
Time: 10:00 AM – 5:00 PM
-
Venue: Four Points by Sheraton, Fatehgunj, Vadodara, Gujarat.
Key Vacancies
-
Technology Transfer Executive: B.Tech/M.Tech Chemical Engineering with 2–8 years in process scale-up and mass balance calculations.
-
Production Supervisor (Kilo Lab/Pilot Plant): M.Sc (2 years exp) or B.Sc/Diploma (5 years exp) to oversee experimental batches and technology absorption.
7. Unison Pharmaceuticals – Quality Control & Engineering
Unison Pharmaceuticals is hiring for its Moraiya (Ahmedabad) plant, with the interview taking place in Vadodara.
Event Schedule
-
Date: 11th January 2026 (Sunday)
-
Time: 10:00 AM – 4:00 PM
-
Venue: Baroda Productivity Council, Productivity House, Alkapuri, Vadodara.
Role Requirements
-
QC Roles: HPLC Analysts, Micro Analysts, and Samplers (1–8 years exp).
-
Engineering Roles: Electricians, Fitters, and Instrumentation Technicians (2–8 years exp).
8. Stallion Laboratories – QC and ADL Specialist Roles
Stallion Laboratories is focused on USFDA-approved OSD facility experienced professionals.
Event Schedule
-
Date: 10th January 2026 (Saturday)
-
Time: 09:00 AM – 02:00 PM
-
Venue: Stallion Laboratories Unit-II, GIDC Industrial Park-II, Vasna Chacharwadi, Ahmedabad.
Vacancies
-
Quality Control Analyst: 20 Vacancies for Sr. Officers/Executives (3–7 years exp) with HPLC and pharmacopoeial updation skills.
-
ADL Analyst: 7 Vacancies for AMV/AMT and analytical documentation specialists.
9. Scott Edil – Sterile Manufacturing Roles
Scott Edil is strengthening its team at the Baddi, Himachal Pradesh facility.
Vacancy Details
-
QA Validation (Sterile): Senior Officer to Manager roles (4–16 years exp).
-
QA IPQA: Officer/Executive roles (2–6 years exp).
-
Production Manager (Sterile): Managing sterile operations (10–15 years exp).
-
Quality Control: M.Sc candidates with 2–8 years in analytical testing.
10. o2h Discovery – Multi-Domain Walk In
o2h Discovery offers a diverse range of roles across EHS, IT, HR, and Admin.
Event Schedule
-
Date: 11th January 2026 (Sunday)
-
Time: 09:00 AM – 03:00 PM
-
Venue: Shivalik Shilp 1, Iskcon Cross Road, Ahmedabad.
Available Positions
-
EHS Specialist: 2–10 years exp (M.Pharm/M.Sc with PDIS).
-
HR Intern/Talent Acquisition: 0–5 years exp (MBA HR).
-
IT & Admin Executives: 2–4 years exp.
Critical Documentation Checklist
All candidates attending the above walk-in interviews must carry the following:
-
Updated Curriculum Vitae (CV).
-
Recent Color Passport-size Photographs.
-
Original and photocopies of Educational Certificates (All semesters).
-
Aadhaar Card and PAN Card.
-
Salary Proof: Last 3 months’ pay slips and latest CTC breakup letter.
-
Experience/Relieving letters from previous employers.
Preparation and Tips for Success
In the 2026 pharmaceutical landscape, regulatory compliance is the top priority. Candidates should be prepared to discuss:
-
Audit Readiness: Experience with USFDA, MHRA, or EU-GMP inspections.
-
Technical Proficiency: Hands-on experience with specific instrumentation (HPLC, GC, PAS X, etc.).
-
Quality Mindset: Understanding of ALCOA+ principles and data integrity.