Pharmaceutical job opportunities across India: Asian Pharma (Baddi), APL Health Care (Jadcherla), Graviti Pharma (Hyderabad) walk-ins, Concord Biotech (Ahmedabad) injectable roles, and Mepro Pharmaceuticals (Vadodara) QC/QA/RA openings. Full role details, qualifications, responsibilities, and application instructions included.
India Pharma Jobs — Asian Pharma · APL Health Care (Aurobindo) · Graviti Pharma · Concord Biotech · Mepro Pharmaceuticals
The pharmaceutical sector in India continues to expand rapidly in 2025, offering abundant opportunities for freshers and experienced professionals across production, quality, regulatory, engineering, warehouse, purchase, and administrative functions. Multiple hiring notices — from urgent mass recruitments to walk-in drives and targeted senior openings — for five companies actively hiring: Asian Pharma (Baddi, Himachal Pradesh), APL Health Care (Aurobindo) (Jadcherla, Telangana), Graviti Pharma (Hyderabad), Concord Biotech (Ahmedabad), and Mepro Pharmaceuticals (Vadodara).
1) Asian Pharma — Multiple Openings (Production / QC / QA / Microbiology / Warehouse / Purchase / Engineering / HR / Admin)
Location: Khasra No. 105/106, Village – Katha, Tehsil – Baddi, Distt Solan, Himachal Pradesh – 173205
Apply: Send resume to hr@asianpharma.in
Contact: 78079-18900
Asian Pharma is conducting an urgent hiring drive spanning entry to mid-senior level roles across multiple departments. These openings support oral solid dosage (OSD) and injectable/packer operations typical of a regulated manufacturing site. Candidates with domain knowledge in GMP, documentation, and hands-on production skills will be preferred.
Open Positions & Requirements
- Quality Control (QC): Executive / Sr. Officer — B.Sc / M.Sc; experience 2–4 years depending on level. Tasks: RM/PM/FP testing, HPLC/wet chemistry, stability testing, documentation.
- Microbiology: Officer — B.Tech; 1–2 years. Tasks: EM sampling, MLT, sterility/BET basics.
- Warehouse: Executive – Packing Material — Graduate; 4–5 years. Tasks: inventory, packing material control, vendor liaison.
- Engineering: Sr. Officer (HVAC), Sr. Officer (Documentation), Jr. Officer, ITI Freshers — Diploma/ITI; 0–3 years. Tasks: HVAC maintenance, equipment documentation, preventive/breakdown maintenance.
- Production (OSD): Officer – Packing — B.Pharm; 1–2 years. Tasks: packing operations, line changeovers, SOP adherence.
- Purchase: Assistant Manager — Graduate; 9–10 years. Tasks: procurement, vendor development, cost control.
- Quality Assurance (QA): IPQA – Sr. Officer (Injection section) — B.Pharm; 3–4 years. Tasks: IPQA protocols, media fill oversight, line clearance.
- HR / Admin: Manager / Assistant Manager — MBA; 12–15 years. Tasks: policy, staffing, labour compliance, HR systems.
What Asian Pharma looks for
- Strong GMP mindset and documentation discipline.
- Demonstrable technical skills for role (e.g., HPLC basics, HVAC troubleshooting, SAP or inventory systems for warehouse).
- Team collaborators who are process-focused, accountable, and disciplined.
- For leadership roles: vendor management, cross-functional communication, and strategic purchasing skills.
How to apply
Email hr@asianpharma.in with an updated CV. Include the position in the subject line and a one-line summary of your experience. Walk-ins may be considered if specified by HR; otherwise contact the number above.

2) APL Health Care (Aurobindo) — Multiple Openings (Production / QA / Engineering)
Location: Unit-1, Polepally, Jadcherla, Telangana
Apply: Email CV to Sankararao.chitikela@Aurobindo.com
APL Health Care, part of the Aurobindo group, seeks professionals experienced in OSD formulations and regulated market requirements (US FDA, MHRA). The site emphasizes immediate joining and candidates with experience in compression, capsule filling and continuous coating systems.
Open Positions
- Production: TA / Sr. TA / Executive — compression and capsule filling experience preferred. (3–7 years)
- Engineering: Executive — continuous coating experience. (3–7 years)
- Quality Assurance: Executive — market complaints handling (3–7 years)
Ideal Candidate Profile
- In-depth exposure to OSD manufacturing lines and validation activities.
- Experience supporting regulatory inspections and handling deviations/CAPA.
- Engineering candidates should have hands-on experience with continuous coating equipment and process controls.
- QA: strong investigation skills for market complaints, product complaint lifecycle, and CAPA tracking.
How to apply
Send CV to Sankararao.chitikela@Aurobindo.com. Mention current CTC, notice period, and relevant certification (if any).
3) Graviti Pharma — Walk In Interview for Freshers (Production Trainee)
Location: Pashamylaram, Hyderabad, Telangana – 502307
Dates: Nov 24–29, 2025
Contacts: HR — Silpa / Laxmikanth
Graviti Pharma’s walk-in is targeted at freshers seeking production entry roles. This is ideal for B.Sc / B.Pharm graduates or diploma holders who want hands-on manufacturing experience.
What they expect
- Willingness to learn core production tasks: granulation, compression, coating, blending, filling, packing.
- Strict adherence to GMP, SOPs, and safety norms.
- Shift flexibility and a team-oriented attitude.
- Basic documentation skills and the ability to follow master batch instructions.
Walk-in Preparation Tips
- Carry updated CV, Aadhaar copy, and qualification certificates.
- Brush up on basic equipment names (RMG, FBD, tablet press, coating pan).
- Be ready to discuss internship projects, college labs or any practical exposure.
Why apply
A great starting point for candidates who want structured on-the-job training and exposure to manufacturing lines. Freshers with discipline and willingness to rotate shifts often fast-track into operator or documentation roles.
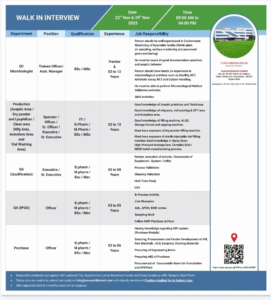
4) Concord Biotech Limited — Freshers & Experienced (QC, QA, Production, Purchase)
Location: Dholka, Ahmedabad, Gujarat
Walk-in Dates: 22 Nov & 29 Nov 2025 | 09:00 AM – 04:00 PM
Apply / Contact: hrinji@concordbiotech.com
Phone: 02714-671222 / +91-7074014306
Concord Biotech is a recognized name in sterile injectables and biopharma. The company is hiring across microbiology, aseptic production, lyophilization, QA/validation, and procurement — catering to both freshers and professionals up to 12 years’ experience.
Roles & Key Responsibilities
- QC Microbiologist (Trainee → Asst Manager): Environmental monitoring, sterility/BET/MLT testing, method validation, culture handling.
- Production (Aseptic/Lyophilizer/Clean Areas): Operate vial washers, filling lines, lyophilizers; execute aseptic techniques; follow BMR.
- QA (Qualification/Validation): Manage DQ/IQ/OQ/PQ, cleaning validation, CSV support.
- IPQA Officer: In-process checks, line clearance, sampling.
- Purchase Officer: Vendor development, ERP purchase module, API/excipient sourcing.
Candidate Requirements
- B.Sc / M.Sc in Microbiology/Life Sciences or B.Pharm / M.Pharm for production/QA roles.
- ITI candidates may be suitable for certain operator roles.
- Strong aseptic technique knowledge and experience with environmental monitoring tools.
Walk-in guidance
Bring updated CV, passport photo, appointment/increment letters, and salary breakup. If you previously interviewed within 3 months, do not reapply.
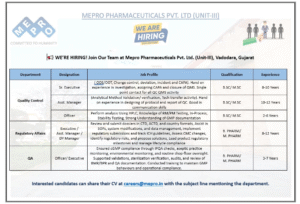
5) Mepro Pharmaceuticals (Unit-III) — QC, QA & Regulatory Affairs
Location: Vadodara, Gujarat
Apply: careers@mepro.in (mention Department & Designation in subject)
Mepro is scaling its QC, QA and Regulatory teams and seeks mid to senior level professionals with WHO-GMP experience. Roles span laboratory leadership, method validation, regulatory dossier preparation, and QA oversight.
Key Openings & Expectations
- QC – Sr. Executive (8–10 yrs): OOS/OOT investigations, CAPA, analytical oversight, HPLC & stability work.
- QC – Asst Manager (10–12 yrs): Method validation, tech transfer, protocol/report design.
- QC – Officer (2–6 yrs): RM/PM/FP testing, documentation.
- Regulatory Affairs (Exec → Dy Manager; 8–12 yrs): CTD/ACTD dossier submissions, CMC assessments, lifecycle management.
- QA (Officer / Executive; 3–7 yrs): IPQA, aseptic monitoring, audit support, BMR/BPR review.
Ideal Candidate Profile
- Strong technical knowledge in HPLC, dissolution, stability program design.
- Proven experience in regulatory submissions and global markets advantageous.
- Excellent documentation skills, investigation capability, and audit readiness experience.
How to apply
Email careers@mepro.in and clearly state the department and role you are applying for in the subject line.
Preparing for Walk-Ins & Interviews — Practical Tips
Whether applying for a trainee operator role or a senior QA/RA position, preparation is key. Here’s a checklist you can use:
Documentation
- Updated CV (one page for freshers, two pages max for experienced).
- Passport-size photos.
- Educational certificates and mark sheets.
- Experience letters, appointment/increment letters, salary slips (for experienced).
- ID proof (Aadhaar, PAN, Passport).
Technical Preparation (by role)
- Production / Operator: Know basic equipment names (RMG, FBD, tablet press), batch record flow, line clearance, basic troubleshooting.
- QC / Microbiology: Be ready to discuss HPLC basics, titrations, KF, sterility test basics, environmental monitoring methods (settle plates, active air sampling).
- QA / IPQA: Understand deviation/CAPA lifecycle, BMR/BPR review checkpoints, media fill concepts, line clearance.
- Engineering: Be prepared to discuss preventive maintenance, HVAC basics, utilities (WFI, compressed air), and breakdown resolution examples.
- Regulatory Affairs: Familiarity with CTD structure, CMC sections, dossier lifecycle, and global submission timelines.
Soft Skills & Behavioural
- Demonstrate teamwork, discipline, and willingness to work shifts.
- Show problem-solving examples from academics or previous roles.
- Communicate clearly and concisely — avoid long monologues.
- For leadership roles, provide examples of project ownership, vendor management, or cross-functional coordination.
Career Progression in Pharma — What to Expect
Starting in operator or QC roles can lead to steady career growth:
- Operator / Production → Senior Operator → Shift In-charge → Production Supervisor → Production Manager.
- QC Analyst → Senior Analyst → QC Lead → QC Manager → Head of QC.
- Microbiology Trainee → Microbiologist → Senior Microbiologist → Microbiology Manager.
- QA/IPQA → QA Executive → QA Manager → Head of Quality.
- Regulatory Affairs: Executive → Manager → Head of Regulatory Affairs — with global mobility for dossier experts.
Certifications (e.g., Six Sigma, Quality Management, Annex-1 training, CSV) and hands-on experience with inspections and tech transfers provide major advantages.




