Extensive career opportunities in Hyderabad’s pharmaceutical and clinical research sectors. Hiring for Quality Assurance, Validation, Clinical Monitoring, and Production roles at Zenotech (Sun Pharma), Hetero, Aurobindo, and Natco Pharma.
The 2026 Pharmaceutical and Clinical Research Career: Hyderabad Hub Opportunities
The pharmaceutical landscape in Hyderabad continues to solidify its position as the “Vaccine Capital” and “Pharmacy of the World” as we enter early 2026. Leading conglomerates and Clinical Research Organizations (CROs) are currently on a massive recruitment drive to fill specialized roles in Quality Assurance, Validation, Clinical Monitoring, and Environmental Health and Safety (EHS). The latest vacancies at top-tier companies including Zenotech Laboratories (Sun Pharma Group), Hetero Labs, Natco Pharma, and Aurobindo Pharma.
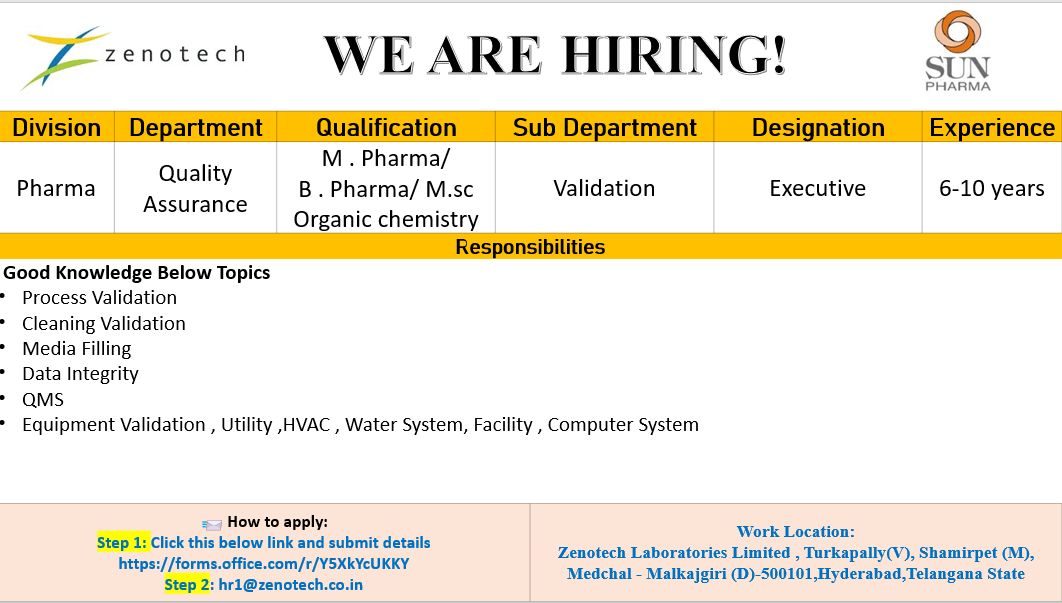
1. Zenotech Laboratories (Sun Pharma Group): QA Validation Excellence
Zenotech Laboratories, a key subsidiary of the Sun Pharma Group, is seeking high-caliber professionals for its Validation department. This role is pivotal in ensuring that all pharmaceutical processes meet the stringent global standards required for high-quality medicine production.
Position: Executive – Validation (Quality Assurance)
-
Experience: 6–8 Years
-
Location: Turkapally, Shamirpet, Hyderabad
-
Core Competencies:
-
Process & Cleaning Validation: Maintaining the integrity of manufacturing cycles and ensuring zero cross-contamination.
-
Aseptic Processing: Expert knowledge of Media Fill operations and sterile environmental controls.
-
Regulatory Compliance: Mastery of Data Integrity (DI) and Quality Management Systems (QMS).
-
Technical Qualification: Validation of HVAC, Water Systems, and Computer System Validation (CSV) under GAMP5 guidelines.
-
Application Procedure
Candidates must follow a dual-step application process:
-
Digital Registration: Submit details via the official Microsoft Form: https://forms.office.com/pages/responsepage.aspx?id=aNogj6LdMkm7D4ANhXmIXYCRpGUsQ-BBoylg3PUr7WVUMDVFOEwyUUE1TElVMFJJTUhRMkJSREg3Ry4u&route=shorturl
-
Email Submission: Forward an updated CV to hr1@zenotech.co.in.
2. Hetero Labs Limited: Leadership in OSD & Parenteral Validation
Hetero Labs, one of India’s largest generic pharmaceutical companies, is looking for experienced managers to spearhead their Qualification and Validation teams at the Jadcherla unit.
Position: Deputy Manager / Manager (QA Validation)
-
Location: Jadcherla, Hyderabad (Unit-V)
-
Specialization: Oral Solid Dosage (OSD) and Parenterals
-
Key Responsibilities:
-
Lifecycle Management: Oversight of DQ, IQ, OQ, and PQ for equipment and utilities.
-
Global Audit Readiness: Ensuring the facility is prepared for inspections by USFDA, MHRA, WHO, and TGA.
-
Deviation Management: Leading investigations into OOS (Out of Specification) and OOT (Out of Trend) results using root cause analysis.
-
-
Contact: Interested leaders should share their profiles with Prashanthkumar.v@hetero.com.
3. Jeevan Scientific Technology: Advancing Clinical Trial Monitoring
Clinical monitoring is the backbone of drug development. Jeevan Scientific, a prominent CRO, is expanding its monitoring team to support diverse clinical trial phases.
Position: CRA / Senior CRA (Clinical Trials)
-
Education: Pharm D or M.Pharm
-
Experience: 4–6+ years of specialized monitoring experience.
-
Expertise Required:
-
Regulatory Knowledge: Deep understanding of ICH-GCP guidelines and FDA/EMA regulations.
-
Site Management: End-to-end responsibility for site selection, initiation, routine monitoring, and close-out visits.
-
Data Integrity: Proficient with Electronic Data Capture (EDC) and eCRF systems to ensure patient safety and protocol compliance.
-
-
Contact: Submit CVs to sharad.chandra@jeevanscientific.com.
4. Natco Pharma Limited: Chemical Division Opportunities
Natco Pharma is hiring for its API (Active Pharmaceutical Ingredient) unit in Mekaguda. They are seeking candidates for two distinct wings of the Quality Assurance department.
A. QA Engineering (API)
-
Position: Chemist / Officer
-
Experience: 4–7 Years
-
Qualification: B.Tech (Mechanical) or M.Sc
-
Focus: Engineering assurance, SOP preparation, and validation of SCADA, PLC, and HMI systems for automated manufacturing.
B. IPQA (In-Process Quality Assurance)
-
Position: Chemist / Officer
-
Experience: 4–7 Years
-
Qualification: M.Sc (Chemistry)
-
Focus: Line clearance, online batch record review, and management of crystallization, filtration, and drying process parameters.
-
Contact: Send applications to Farooq.md@natcopharma.co.in.
5. Granules India: Specialized Site Investigation (SIT)
Granules India is looking for a senior professional to lead their Site Investigation Team (SIT) for the Gagillapur Formulation OSD plant.
Position: Deputy Manager (SIT – QA)
-
Experience: 9–12 Years
-
Qualification: B.Pharm / M.Pharm / M.Sc
-
Role Focus: This is a highly specialized role requiring mandatory experience in investigating lab failures and data integrity issues. The candidate will collaborate with Cross-Functional Teams (CFTs) to drive CAPA effectiveness.
-
Contact: Reach out to sandhya.bathina@granulesindia.com.
6. Aurobindo Pharma: Mega Walk-In Drive
Aurobindo Pharma is hosting a large-scale recruitment event for its Unit-III facility in Bachupally. This is an excellent opportunity for entry-level and mid-level professionals.
Walk-In Details
-
Date: 01 February 2026 (Sunday)
-
Time: 09:30 AM – 02:00 PM
-
Location: Unit-III, Bachupally, Hyderabad
-
Departments: Production, Packing, QC, and QA
-
Positions: Technical Assistant / Executive
-
Experience: 1–5 Years
-
Education: ITI, Diploma, B.Sc, M.Sc, B.Pharm, M.Pharm
7. Lupin Limited: EHS Leadership in API
Safety is paramount in chemical manufacturing. Lupin is hiring for an Environmental Health and Safety (EHS) role to manage risk and compliance.
Position: EHS Executive
-
Location: Pithampur, Madhya Pradesh
-
Salary: ₹7 LPA – ₹8 LPA
-
Key Tasks: Risk assessments, HAZOP, safety training, and fire safety standards.
-
Contact: Share resumes with suhailkhan1@lupin.com or babitamanaware@lupin.com.
8. Advity Research: Clinical Career Entry
Advity Research is opening doors for freshers and experienced clinical professionals in the heart of Hyderabad.
Roles Available
-
Pharmacist, CRA, Analyst, and Custodian
-
Experience: 0–5 Years
-
Work Mode: Full-time, On-premise
-
Contact: hr@advityresearch.com
Strategic Advice for Job Seekers in 2026
To stand out in the competitive Hyderabad pharma market:
-
Certification: For QA roles, certifications in Six Sigma or Lean Manufacturing add immense value.
-
Digital Literacy: Proficiency in SAP and eQMS (Electronic Quality Management Systems) is no longer optional; it is a prerequisite for roles at companies like Natco and Granules.
-
Audit Awareness: Familiarize yourself with the latest USFDA Warning Letters and 483s to understand current regulatory trends.